Triazavirin
A guanine nucleotide analogue.
General information
Triazavirin is a guanine nucleotide analogue displaying anti-influenza activity (DrugBank).
Triazavirin on PubChem
Triazavirin on Wikipedia
Synonyms
TZV; Riamilovir
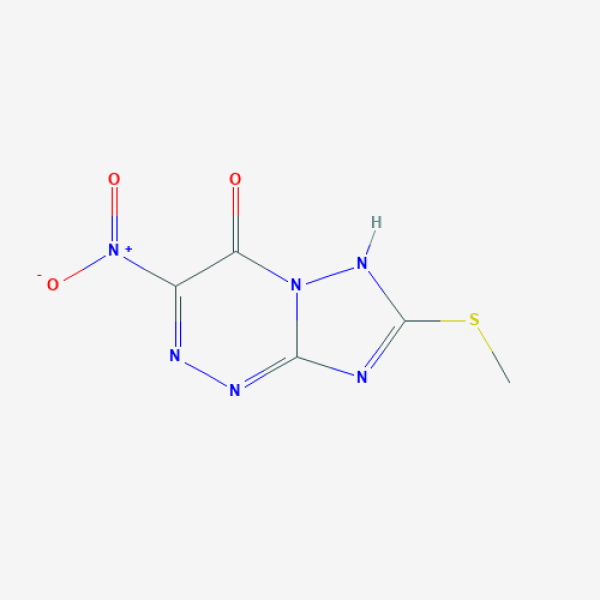
CSC1=NC2=NN=C(C(=O)N2N1)[N+](=O)[O-]
Supporting references
| Link | Tested on | Impact factor | Notes | Publication date |
|---|---|---|---|---|
|
Efficacy and Safety of Triazavirin Therapy for Coronavirus Disease 2019: A Pilot Randomized Controlled Trial
Small molecule Randomized controlled double-blind trial |
Patients | 6.50 | No-statistically significant improvement in the Triazavirin-treated group within the primary outcome. Numerically, more patients in the treated group reached the primary outcome and less frequently needed the usage of certain additional medication, however. Sample size: 26 + 26 placebo. Dosage: 250 mg tree to four times a day for 7 days. Endpoint: Clinical improvement (normalization of body temperature, respiratory rate, oxygen saturation, cough, and absorption of pulmonary infection by chest computed tomography (CT) until 28 days after randomization) (primary outcome). |
Sep/08/2020 |
AI-suggested references
Clinical trials
| ID | Title | Status | Phase | Start date | Completion date |
|---|---|---|---|---|---|
| NCT04973462 | Evaluation of The Efficacy of Triazavirin Versus Oseltamivir in Egyptian Patients Infected With COVID-19 | Recruiting | Phase 4 | Aug/01/2021 | Dec/30/2021 |
|
|||||
| NCT04581915 | PHRU CoV01 A Trial of Triazavirin (TZV) for the Treatment of Mild-moderate COVID-19 | Recruiting | Phase 2|Phase 3 | Sep/08/2020 | Dec/31/2021 |
|
|||||
