Toremifene
A selective estrogen receptor modulator.
General information
Toremifene is a selective oestrogen receptor modulator (NCIt) used for treatment of oestrogen receptor-positive metastatic breast cancer in postmenstrual women (HMDB).
Toremifene on DrugBank
Toremifene on PubChem
Toremifene on Wikipedia
Marketed as
ACAPODENE; FARESTON; TOREMIFENE CITRATE
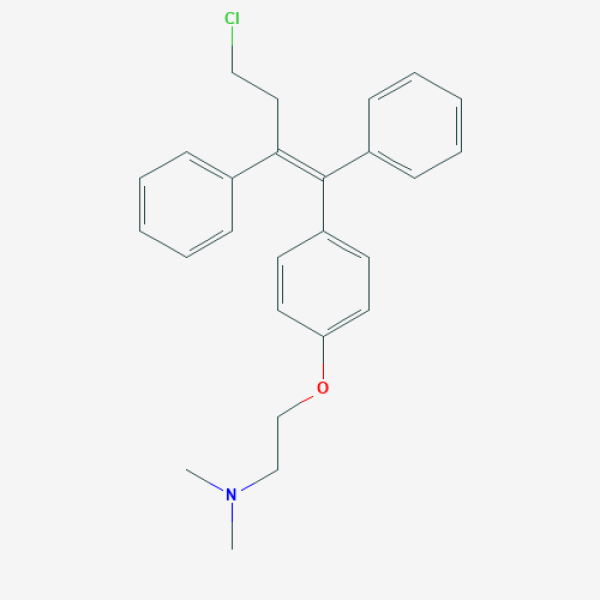
CN(C)CCOC1=CC=C(C=C1)/C(=C(/CCCl)\C2=CC=CC=C2)/C3=CC=CC=C3
Supporting references
| Link | Tested on | Impact factor | Notes | Publication date |
|---|---|---|---|---|
|
Network-based drug repurposing for novel coronavirus 2019-nCoV/SARS-CoV-2
|
in silico | in combination with emodin |
Mar/16/2020 | |
|
Human organs-on-chips as tools for repurposing approved drugs as potential influenza and COVID19 therapeutics in viral pandemics
|
human organ-on-a-chip | Apr/14/2020 | ||
|
Repurposing of FDA-Approved Toremifene to Treat COVID-19 by Blocking the Spike Glycoprotein and NSP14 of SARS-CoV-2
3CLpro nsp14 Cancer Small molecule In silico |
adenocarcinomic human alveolar basal epithelial cells | 4.07 | Predicted to inhibit the SARS-CoV-2 spike protein and the nsp14 methyltrasnsferase. |
Sep/09/2020 |
|
Identification of SARS-CoV-2 entry inhibitors among already approved drugs
Small molecule In vitro Screening |
Huh-7 cells (pseudovirus-based assay); Vero E6 cells (antiviral activity); SARS-CoV-2 (WIV04) | 5.06 | Inhibits SARS-CoV-2 spike protein pseudovirus cell entry and significally inhibits viral replication in vitro. |
Oct/28/2020 |
Clinical trials
| ID | Title | Status | Phase | Start date | Completion date |
|---|---|---|---|---|---|
| NCT04531748 | Selective Estrogen Modulation and Melatonin in Early COVID-19 | Withdrawn | Phase 2 | Dec/01/2021 | Sep/01/2022 |
|
|||||
