Topotecan
A topoisomerase I inhibitor.
General information
Topotecan is an FDA-approved TOP1 topoisomerase inhibitor. The siRNA knockdown of TOP1 has been shown to suppress the upregulation of several genes associated with SARS-CoV-2 infection progression, several of which are involved in inflammatory responses. Similarly, topotecan treatment experimentally dampened expression of several pro-inflammatory genes in vitro and in lungs of a hamster model after a viral challenge. The drug also reduced lung pathology. In a mouse model, early and intermediate treatment did not reduce mortality due to the drug’s mechanism of action. On the other hand, late treatment significantly increased survival of the infected mice compared to control (Ho et al., 2021).
Topotecan on DrugBank
Topotecan on PubChem
Topotecan on Wikipedia
Marketed as
HYCAMTIN; POTACTASOL; TOPOTECAN
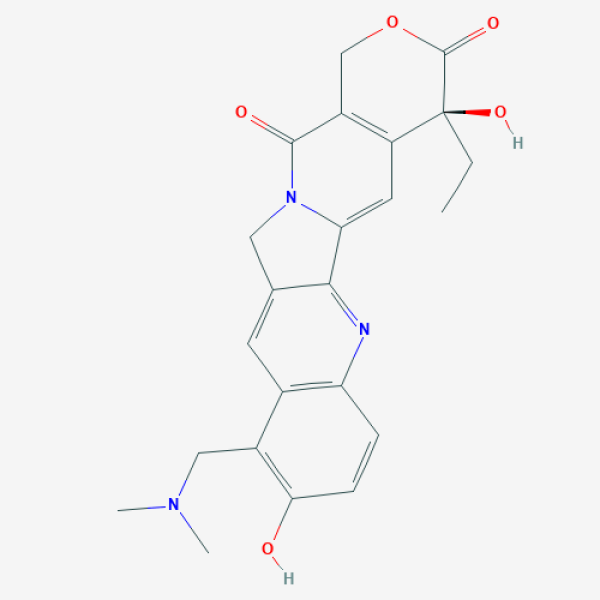
CC[C@@]1(C2=C(COC1=O)C(=O)N3CC4=CC5=C(C=CC(=C5CN(C)C)O)N=C4C3=C2)O
Supporting references
| Link | Tested on | Impact factor | Notes | Publication date |
|---|---|---|---|---|
|
TOP1 inhibition therapy protects against SARS-CoV-2-induced lethal inflammation
Small molecule Animal model In vitro Mechanism |
A549-ACE2 cells; Vero E6 cells; B6.Cg-Tg(K18-866 ACE2)2Prlmn/J mice; golden Syrian hamsters; SARS-CoV-2 isolates USA-WA1/2020 and HKG/13_P2/2020 | 38.64 | Topotecan treatment dampened expression of several pro-inflammatory genes in vitro and in lungs of a hamster model after a viral challenge. The drug also reduced lung pathology. In a mouse model, early and intermediate treatment did not reduce mortality due to the drug’s mechanism of action. On the other hand, late treatment significantly increased survival of the infected mice compared to control. |
Mar/30/2021 |
AI-suggested references
| Link | Publication date |
|---|---|
|
In silico identification of available drugs targeting cell surface BiP to disrupt SARS-CoV-2 binding and replication: Drug repurposing approach
|
Feb/04/2020 |
Clinical trials
| ID | Title | Status | Phase | Start date | Completion date |
|---|---|---|---|---|---|
| NCT05083000 | Reducing Hypoxia in Patients With Coronavirus Disease (COVID-19) Using Topotecan With Standard of Care | Recruiting | Phase 1 | Aug/16/2021 | Mar/31/2022 |
|
|||||
