TMC-310911
An HIV-1 protease inhibitor.
General information
TMC-310911 on PubChem
Synonyms
ASC09; ASC-09; (3r,3As,6ar)-Hexahydrofuro[2,3-B]furan-3-Yl {(2s,3r)-4-[({2-[(1-Cyclopentylpiperidin-4-Yl)amino]-1,3-Benzothiazol-6-Yl}sulfonyl)(2-Methylpropyl)amino]-3-Hydroxy-1-Phenylbutan-2-Yl}carbamate
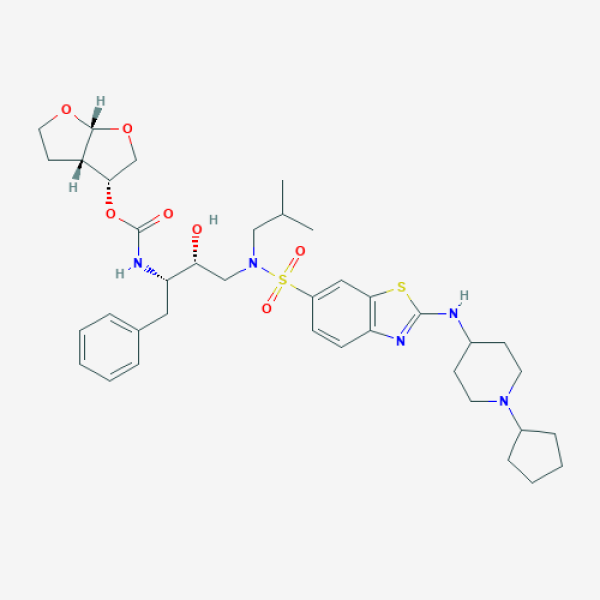
CC(C)CN(C[C@H]([C@H](CC1=CC=CC=C1)NC(=O)O[C@H]2CO[C@@H]3[C@H]2CCO3)O)S(=O)(=O)C4=CC5=C(C=C4)N=C(S5)NC6CCN(CC6)C7CCCC7
Supporting references
| Link | Tested on | Impact factor | Notes | Publication date |
|---|---|---|---|---|
|
Rational approach toward COVID-19 main protease inhibitors via molecular docking, molecular dynamics simulation and free energy calculation
3CLpro Small molecule In silico |
in silico | 4.00 | Predicted to inhibit the SARS-CoV-2 3C-like protease. |
Oct/19/2020 |
|
Molecular modeling evaluation of the binding effect of five protease inhibitors to COVID-19 main protease
3CLpro Small molecule In silico |
in silico | 1.77 | Predicted to inhibit the SARS-CoV-2 3C-like protease. |
Dec/11/2020 |
|
In Silico Evaluation of Prospective Anti-COVID-19 Drug Candidates as Potential SARS-CoV-2 Main Protease Inhibitors
3CLpro Small molecule In silico |
in silico | 1.32 | Predicted to inhibit the SARS-CoV-2 3C-like protease. |
Jan/02/2021 |
Clinical trials
| ID | Title | Status | Phase | Start date | Completion date |
|---|---|---|---|---|---|
| NCT04261907 | Evaluating and Comparing the Safety and Efficiency of ASC09/Ritonavir and Lopinavir/Ritonavir for Novel Coronavirus Infection | Unknown status | Not Applicable | Feb/07/2020 | Jun/30/2020 |
|
|||||
| NCT04261270 | A Randomized,Open,Controlled Clinical Study to Evaluate the Efficacy of ASC09F and Ritonavir for 2019-nCoV Pneumonia | Unknown status | Phase 3 | Feb/01/2020 | Jul/01/2020 |
|
|||||
