Thalidomide
A piperidinyl isoindole.
General information
Thalidomide is a piperidinyl isoindole compound with anti-inflammatory, immunomodulatory, anti-angiogenic, and anti-neoplastic properties. It is used for treatment of multiple myeloma or cutaneous manifestations of leprosy. It is teratogenic, however (DrugBank, PubChem). It is on the World Health Organization Model List of Essential Medicines.
Thalidomide on Wikipedia
Marketed as
CONTERGAN; DISTAVAL; K-17; PRO-BAN M; SEDALIS; SOFTENON; TALIMOL; THALED; THALIDOMIDE; CELGENE; THALIDOMID
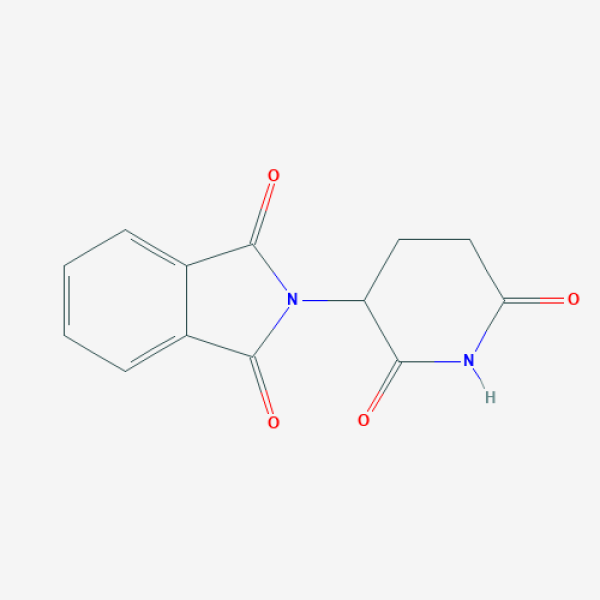
C1CC(=O)NC(=O)C1N2C(=O)C3=CC=CC=C3C2=O
Supporting references
| Link | Tested on | Impact factor | Notes | Publication date |
|---|---|---|---|---|
|
Thalidomide Combined with Short-term Low-Dose Glucocorticoid Therapy for the Treatment of Severe COVID-19: A Case-Series Study
Severe severity Small molecule Critical severity Case series |
Severe to critical COVID-19 patients | 3.20 | Observed faster SARS-CoV-2 negative conversion, shorter length of hospital stay, or reduced need of mechanical ventilation in critical COVID-19 patients. Treatment with thalidomide, together with low-dose glucocorticoid therapy, was suggested to be effective in improving critical COVID-19 patients' prognosis. It could act via cytokine production suppression. Sample size: 6 + 6 control. Dosage: 100 mg daily for 7+ days. |
Dec/10/2020 |
AI-suggested references
Clinical trials
| ID | Title | Status | Phase | Start date | Completion date |
|---|---|---|---|---|---|
| NCT04273529 | The Efficacy and Safety of Thalidomide in the Adjuvant Treatment of Moderate New Coronavirus (COVID-19) Pneumonia | Unknown status | Phase 2 | Feb/20/2020 | Jun/30/2020 |
|
|||||
| NCT04273581 | The Efficacy and Safety of Thalidomide Combined With Low-dose Hormones in the Treatment of Severe COVID-19 | Unknown status | Phase 2 | Feb/18/2020 | May/30/2020 |
|
|||||
