Sofosbuvir
An antiviral nucleoside analogue.
General information
Sofosbuvir is a prodrug and a nucleoside analogue that inhibits hepatitis C virus RNA-dependent RNA polymerase (NCIt). It might inhibit the SARS-CoV-2 RNA-dependent RNA polymerase at the early stages of viral replication (Sacramento et al., 2021).
Sofosbuvir on DrugBank
Sofosbuvir on PubChem
Sofosbuvir on Wikipedia
Marketed as
SOVALDI
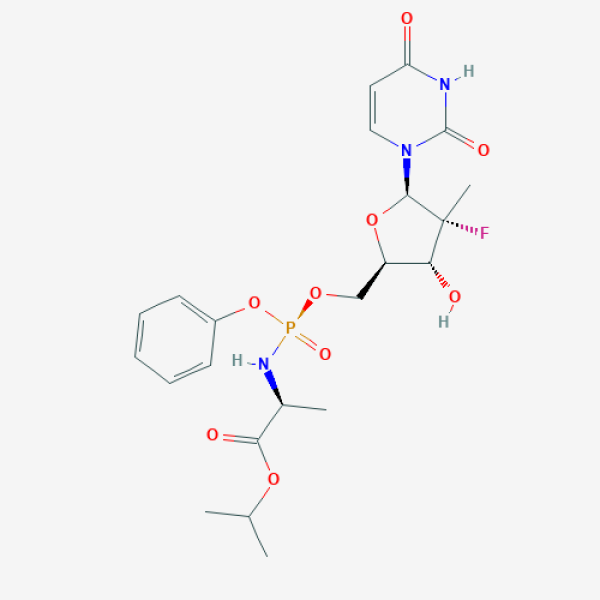
C[C@@H](C(=O)OC(C)C)N[P@](=O)(OC[C@@H]1[C@H]([C@@]([C@@H](O1)N2C=CC(=O)NC2=O)(C)F)O)OC3=CC=CC=C3
Supporting references
| Link | Tested on | Impact factor | Notes | Publication date |
|---|---|---|---|---|
|
Anti-HCV, nucleotide inhibitors, repurposing against COVID-19.
|
in silico | Feb/28/2020 | ||
|
Sofosbuvir protects human brain organoids against SARS-CoV-2
Preprint |
iPSC-derived human brain organoids | May/31/2020 | ||
|
The in vitro antiviral activity of the anti-hepatitis C virus (HCV) drugs daclatasvir and sofosbuvir against SARS-CoV-2
Preprint |
HuH-7 and Calu-3 cells | Jun/16/2020 | ||
|
Sofosbuvir and daclatasvir compared with standard of care in the treatment of patients admitted to hospital with moderate or severe coronavirus infection (COVID-19): a randomized controlled trial
Severe severity Small molecule Non-randomized controlled open trial Moderate severity |
Patients | 5.44 | The fixed-dose with daclatasvir significantly shortened median duration of hospitalization versus standard care. Sample size: 33 + 33 control. Dosage: 400 mg sofosbuvir and 60 mg daclatasvir in addition to standard care for 14 days. Endpoint: Clinical recovery within 14 days of enrolment (primary). |
Aug/19/2020 |
|
The impact of sofosbuvir/daclatasvir or ribavirin in patients with severe COVID-19
Small molecule Non-randomized controlled open trial |
Patients | 5.44 | In a fixed-dose with daclatasvir significantly reduced time to clinical improvement and caused less side effects when compared to ribavirin treatment. Sample size: 35 + 27 (ribavirin arm). Dosage: 400 mg sofosbuvir and 60 mg daclatasvir twice a day for a maximum of 14 days. |
Aug/18/2020 |
|
MCCS: a novel recognition pattern-based method for fast track discovery of anti-SARS-CoV-2 drugs
3CLpro Small molecule In silico |
in silico | 8.99 | Predicted to inhibit the SARS-CoV-2 3C-like protease. |
Oct/20/2020 |
AI-suggested references
Clinical trials
| ID | Title | Status | Phase | Start date | Completion date |
|---|---|---|---|---|---|
| NCT04561063 | COVID-19 Prophylaxis South Africa (COVER HCW) | Recruiting | Phase 2 | Dec/08/2020 | Feb/28/2022 |
|
|||||
| NCT04443725 | Efficacy and Safety of Anti HCV Drugs in the Treatment of COVID-19 | Not yet recruiting | Phase 2|Phase 3 | Jul/01/2020 | Dec/01/2020 |
|
|||||
| NCT04497649 | Sofosbuvir Containing Regimens in Treatment of COVID 19 Patients | Recruiting | Phase 2|Phase 3 | Jul/01/2020 | Apr/10/2021 |
|
|||||
| NCT04498936 | Sofosbuvir/Ledipasvir and Nitazoxanide for Treatment of COVID-19 | Completed | Phase 4 | Jul/15/2020 | Oct/30/2020 |
|
|||||
| NCT04460443 | Sofosbuvir in Treatment of COVID 19 | Recruiting | Phase 2|Phase 3 | Aug/01/2020 | Aug/31/2021 |
|
|||||
| NCT04773756 | Evaluation of Sofosbuvir and Daclatasvir Combo in COIVD-19 Patients in Egypt | Completed | Phase 4 | Nov/01/2020 | Dec/12/2020 |
|
|||||
| NCT04535869 | Efficacy and Safety of Direct Anti HCV Drugs in the Treatment of SARS-COV-2 (COVID-19) | Recruiting | Phase 3 | Dec/28/2020 | Sep/03/2021 |
|
|||||
| NCT04532931 | COVID-19 Treatment in South Africa | Completed | Phase 2 | Sep/03/2020 | Aug/23/2021 |
|
|||||
| NCT04530422 | Efficacy of Sofosbuvir Plus Ledipasvir in Egyptian Patients With COVID-19 Compared to Standard Treatment | Completed | Phase 3 | Apr/15/2020 | Jul/23/2020 |
|
|||||
