Sildenafil
A phosphodiesterase type 5 inhibitor.
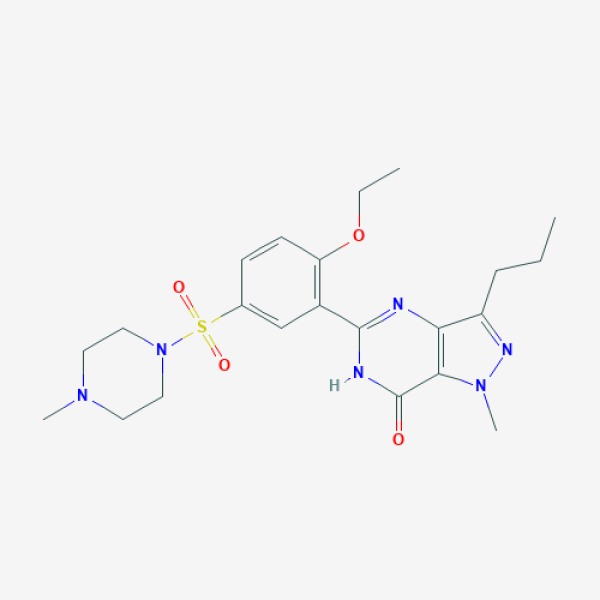
CCCC1=NN(C2=C1N=C(NC2=O)C3=C(C=CC(=C3)S(=O)(=O)N4CCN(CC4)C)OCC)C
Supporting references
| Link | Tested on | Impact factor | Notes | Publication date |
|---|---|---|---|---|
|
Molecular Features of Non-Selective Small Molecule Antagonists of the Bradykinin Receptors
Small molecule In silico |
in silico | 4.29 | Predicted to non-selectively bind bradykinin receptors, which was theorised to alleviate SARS-CoV-2-related inflammation. |
Sep/21/2020 |
AI-suggested references
Clinical trials
| ID | Title | Status | Phase | Start date | Completion date |
|---|---|---|---|---|---|
| NCT04489446 | Sildenafil in COVID-19 | Completed | Phase 1|Phase 2 | Aug/19/2020 | Jun/30/2021 |
|
|||||
| NCT04304313 | A Pilot Study of Sildenafil in COVID-19 | Unknown status | Phase 3 | Feb/09/2020 | Nov/09/2020 |
|
|||||
