Rosuvastatin
A statin.
General information
Rosuvastatin counters lipidemia and displays antineoplastic activities (NCIt).
Rosuvastatin on DrugBank
Rosuvastatin on PubChem
Rosuvastatin on Wikipedia
Marketed as
ASTENDE; CIRANTAN; CRESADEX; CRESTOR; EZALLOR SPRINKLE; PROVISACOR; RAZEL; ROSEDEX; ROSIMOL; ROSUMED; ROSUSTATIN; ROSUVAS; ROSUVAST; ROSVEL; ROVARTAL; ROSUVASTATIN; SIMESTAT; SINLIP; VISACOR; VIVACOR
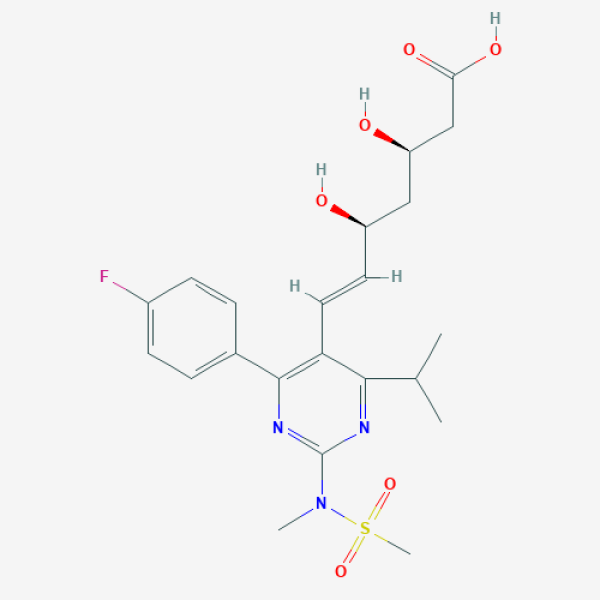
CC(C)C1=NC(=NC(=C1/C=C/[C@H](C[C@H](CC(=O)O)O)O)C2=CC=C(C=C2)F)N(C)S(=O)(=O)C
Supporting references
| Link | Tested on | Impact factor | Notes | Publication date |
|---|---|---|---|---|
|
In-Hospital Use of Statins Is Associated with a Reduced Risk of Mortality among Individuals with COVID-19
|
Patients | 21.57 | A retrospective cohort study of in-hospital statin use among COVID-19 patients. Lower risk of all-cause mortality. Lower use of invasive mechanical ventilation, ARDS prevalence and ICU admission. Possible due to inflammatory response amelioration. Dosage - daily equivalent dose of statin, median 20 mg. Endpoints - 28-day all-cause mortality; ICU admission; use of invasive mechanical ventilation; ARDS incidence. Sample size 190 patients (1,219 statins + 12,762 control). |
Aug/04/2020 |
|
Effectiveness of rosuvastatin plus colchicine, emtricitabine/tenofovir and combinations thereof in hospitalized patients with COVID-19: a pragmatic, open-label randomized trial
ARDS Small molecule Randomized controlled open trial |
Pneumonia patients | The combined treatment using colchicine, emtricitabine, tenofovir, and rosuvastatin resulted in lower mortality and invasive mechanical ventilation need compared to control. Sample size: (ITT) 159 + 161 control. Dosage: 40 mg daily for 14 days. Main outcome: 28-day mortality. |
Dec/20/2021 |
AI-suggested references
Clinical trials
| ID | Title | Status | Phase | Start date | Completion date |
|---|---|---|---|---|---|
| NCT04472611 | Colchicine/Statins for the Prevention of COVID-19 Complications (COLSTAT) Trial | Recruiting | Phase 3 | Oct/30/2020 | Aug/01/2022 |
|
|||||
| NCT04359095 | Effectiveness and Safety of Medical Treatment for SARS-CoV-2 (COVID-19) in Colombia | Completed | Phase 2|Phase 3 | Aug/18/2020 | Jun/30/2021 |
|
|||||
