Ribavirin
A ribonucleoside analogue.
General information
Ribavirin is a synthetic nucleoside analogue. It inhibits viruses (including hepatitis C virus) through incorporation into viral RNA (NCIt). It was computationally modelled to interact with the SARS-CoV-2 RNA-dependent RNA polymerase's active site, but only to weakly base-pair with complementary nucleotides (Byléhn et al., 2021).
Ribavirin on DrugBank
Ribavirin on PubChem
Ribavirin on Wikipedia
Marketed as
COPEGUS; IBAVYR; MODERIBA; REBETOL; REBRETRON; RIBAMIDE; RIBASPHERE; VILONA; VIRAMID; VIRAZIDE; VIRAZOLE
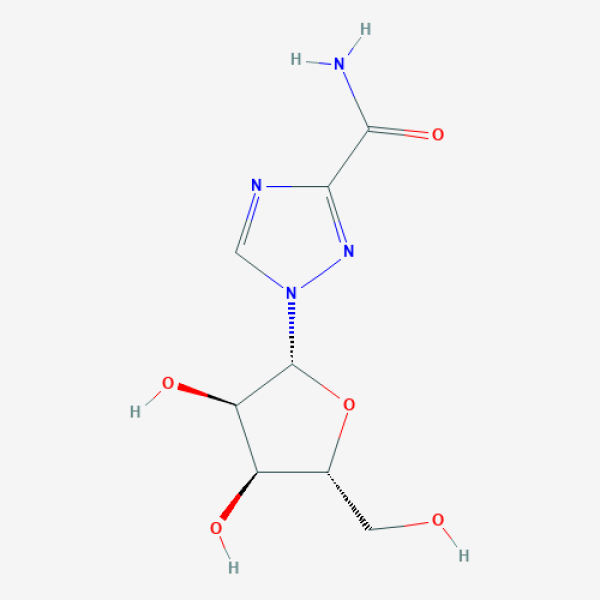
C1=NC(=NN1[C@H]2[C@@H]([C@@H]([C@H](O2)CO)O)O)C(=O)N
Supporting references
| Link | Tested on | Impact factor | Notes | Publication date |
|---|---|---|---|---|
|
Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro
|
VERO E6 cell cultures | High concentrations were required to reduce the viral infection |
Feb/04/2020 | |
|
Anti-HCV, nucleotide inhibitors, repurposing against COVID-19.
|
in silico | Feb/28/2020 | ||
|
Discovering drugs to treat coronavirus disease 2019 (COVID-19).
|
in silico | Intravenous infusion, 500 mg each time, 2 to 3 times/day in combination with IFN-α or lopinavir/ritonavir |
Feb/22/2020 | |
|
No Clear Benefit to the Use of Corticosteroid as Treatment in Adult Patients with Coronavirus Disease 2019 : A Retrospective Cohort Study
|
Patients | in combination with arbidol |
Apr/24/2020 | |
|
Triple combination of interferon beta-1b, lopinavir–ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial
|
Patients | Combination of lopinavir 400 mg and ritonavir 100 mg every 12 h, ribavirin 400 mg every 12 h, and three doses of 8 million international units of interferon beta-1b on alternate days is better than lopinavir-ritonavir in alleviating symptoms and shortening the duration of viral shedding and hospital stay in patients with mild to moderate COVID-19 |
May/08/2020 | |
|
A SARS-CoV-2 protein interaction map reveals targets for drug repurposing
Small molecule |
in silico | 42.78 | Apr/30/2020 | |
|
Modeling the Binding Mechanism of Remdesivir, Favilavir, and Ribavirin to SARS-CoV-2 RNA-Dependent RNA Polymerase
RdRpol Small molecule Mechanism In silico |
in silico | 12.69 | Ribavirin was computationally modelled to bind at the SARS-CoV-2 RNA-dependent RNA polymerase active site. In contrast to remdesivir, it was predicted not to base-pair significantly with the complementary RNA strand. |
Jan/06/2021 |
|
Effect of a combination of Nitazoxanide, Ribavirin and Ivermectin plus zinc supplement (MANS.NRIZ study) on the clearance of mild COVID‐19
Small molecule Non-randomized controlled open trial Phase I clinical trial Mild severity |
Mild COVID-19 patients | 2.02 | In combination with nitazoxanide, ivermectin, and zinc supplementation. Observed improvement in SARS-CoV-2 nasopharyngeal viral clearance at days 7 and 15 compared to control. Sample size: 62 + 51 control. Dosage: 1200 mg. |
Feb/16/2021 |
|
Ribavirin shows antiviral activity against SARS-CoV-2 and downregulates the activity of TMPRSS2 and the expression of ACE2 In Vitro
Small molecule In vitro In silico |
in silico; Vero E6 cells; Caco-2 cells; SARS-CoV-2 isolate Ank1 | 1.95 | The drug inhibited SARS-CoV-2 infection in Vero E6 cells with an IC50 of ca. 0.8 μM. It decreased TMPRSS2 expression (mRNA), protein levels and enzymatic activity in vitro. A decrease of ACE2 levels was observed in Caco-2 cells but not in Vero E6 cells. |
Mar/09/2021 |
|
In silico evaluation of potential inhibitory activity of remdesivir, favipiravir, ribavirin and galidesivir active forms on SARS-CoV-2 RNA polymerase
RdRpol Small molecule In silico |
in silico | 2.01 | The triphosphate active metabolite was predicted to inhibit SARS-CoV-2 RNA-dependent RNA polymerase. |
Mar/25/2021 |
AI-suggested references
Clinical trials
| ID | Title | Status | Phase | Start date | Completion date |
|---|---|---|---|---|---|
| NCT04494399 | IFN Beta-1b and Ribavirin for Covid-19 | Recruiting | Phase 2 | Jul/29/2020 | Aug/01/2022 |
|
|||||
| NCT04828564 | Efficacy and Safety of Favipiravir and Ribavirin Formulation for Treatment of COVID-19 | Not yet recruiting | Phase 2|Phase 3 | Apr/01/2021 | Dec/31/2021 |
|
|||||
| NCT04605588 | A Triple Combination Antiviral Coronavirus Therapy (TriACT) for COVID-19 | Terminated | Phase 2 | Dec/02/2020 | Feb/04/2021 |
|
|||||
| NCT04959786 | MANS-NRIZ Trial for COVID-19 Treatment : Extension Study | Recruiting | Phase 2|Phase 3 | Apr/01/2021 | Dec/01/2022 |
|
|||||
| NCT04276688 | Lopinavir/ Ritonavir, Ribavirin and IFN-beta Combination for nCoV Treatment | Completed | Phase 2 | Feb/10/2020 | Mar/31/2020 |
|
|||||
| NCT04563208 | Study of Oral Administration of Ribavirin and Nitazoxamide Versus Placebo in COVID-19 | Recruiting | Phase 2 | Dec/09/2020 | Feb/28/2022 |
|
|||||
| NCT04664010 | Efficacy and Safety of High-dose Vitamin C Combined With Chinese Medicine Against Coronavirus Pneumonia (COVID-19) | Active, not recruiting | Not Applicable | Feb/06/2020 | Jan/31/2021 |
|
|||||
| NCT04551768 | Study to Evaluate the Safety and Efficacy of VIRAZOLE® in Hospitalized Adult Participants With Respiratory Distress Due to COVID-19 | Completed | Phase 1 | Feb/10/2021 | Aug/17/2021 |
|
|||||
| NCT04392427 | New Antiviral Drugs for Treatment of COVID-19 | Not yet recruiting | Phase 3 | Oct/01/2020 | May/01/2022 |
|
|||||
| NCT04356677 | Study to Evaluate the Safety and Efficacy of VIRAZOLE® in Hospitalized Adult Participants With Respiratory Distress Due to COVID-19 | Withdrawn | Phase 1 | May/01/2021 | Aug/01/2021 |
|
|||||
