Rapamycin
A natural macrocyclic lactone and inhibitor of mammalian Target Of Rapamycin complex 1 (mTORC1).
General information
Rapamycin is a natural lactone compound that inhibits the mammalian target of rapamycin (mTOR), which is a pleiotropic regulatory kinase. It has (among other) immunosuppressive properties (NCIt). Rapamycin was suggested to inhibit SARS-CoV-2 infection via mTOR inhibition (although by an unknown mechanism) (Mullen et al., 2021).
Rapamycin on DrugBank
Rapamycin on PubChem
Rapamycin on Wikipedia
Synonyms
Sirolimus
Marketed as
RAPAMUNE
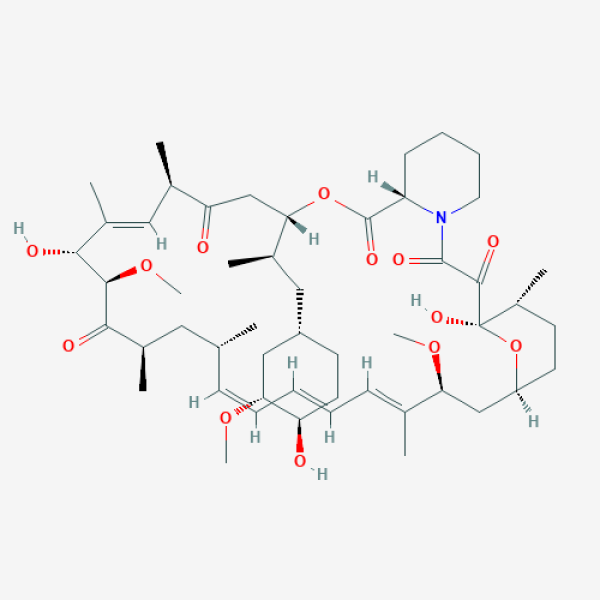
C[C@@H]1CC[C@H]2C[C@@H](/C(=C/C=C/C=C/[C@H](C[C@H](C(=O)[C@@H]([C@@H](/C(=C/[C@H](C(=O)C[C@H](OC(=O)[C@@H]3CCCCN3C(=O)C(=O)[C@@]1(O2)O)[C@H](C)C[C@@H]4CC[C@H]([C@@H](C4)OC)O)C)/C)O)OC)C)C)/C)OC
Supporting references
| Link | Tested on | Impact factor | Notes | Publication date |
|---|---|---|---|---|
|
Network-based drug repurposing for novel coronavirus 2019-nCoV/SARS-CoV-2
|
in silico | in combination with dactinomycin |
Mar/16/2020 | |
|
A SARS-CoV-2 protein interaction map reveals targets for drug repurposing
Small molecule |
in silico | 42.78 | Apr/30/2020 | |
|
Identification of potential treatments for COVID-19 through artificial intelligence-enabled phenomic analysis of human cells infected with SARS-CoV-2
Preprint |
human renal cortical epithelial cells | Apr/23/2020 | ||
|
Repurposing of the approved small molecule drugs in order to inhibit SARS-CoV-2 S protein and human ACE2 interaction through virtual screening approaches
Spike protein Small molecule In silico |
in silico | 3.22 | Predicted to inhibit the SARS-CoV-2 spike protein binding to the host's ACE2 receptor. |
Sep/24/2020 |
|
Drug repurposing for COVID-19 using machine learning and mechanistic models of signal transduction circuits related to SARS-CoV-2 infection
Protein factor Small molecule Antibody In silico |
in silico (machine learning) | 13.49 | Considered by the authors to be among the most relevant drugs identified in a machine-learning algorithm-based screening of compounds which considers causal protein-protein interactions, known drug targets, and specific signalling circuits in <a href= |
Dec/11/2020 |
|
SARS-CoV-2 infection rewires host cell metabolism and is potentially susceptible to mTORC1 inhibition
Small molecule In vitro |
Vero cells; human lung air-liquid interface cultures; SARS-CoV-2 isolate USA-WA1/2020 | 12.12 | Inhibited SARS-CoV-2 replication in Vero cells and human lung air-liquid interface cultures, possibly through mTORC1 inhibition. Rapamycin was the most potent SARS-CoV-2 inhibitor from the mTORC1 inhibitors tested. |
Mar/25/2021 |
AI-suggested references
Clinical trials
| ID | Title | Status | Phase | Start date | Completion date |
|---|---|---|---|---|---|
| NCT04948203 | Assessing the Efficacy of Sirolimus in Patients With COVID-19 Pneumonia for Prevention of Post-COVID Fibrosis | Recruiting | Phase 2|Phase 3 | Jul/09/2021 | Jun/01/2022 |
|
|||||
| NCT04371640 | Sirolimus in COVID-19 Phase 1 | Withdrawn | Phase 1 | Jul/06/2020 | Jul/30/2021 |
|
|||||
| NCT04461340 | Efficacy and Safety of Sirolimus in COVID-19 Infection | Recruiting | Phase 2 | Aug/15/2020 | Nov/30/2020 |
|
|||||
| NCT04482712 | Effects of mTOR Inhibition With Sirolimus (RAPA) in Patients With COVID-19 to Moderate the Progression of ARDS | Withdrawn | Phase 1|Phase 2 | Apr/01/2021 | Jan/01/2023 |
|
|||||
| NCT04374903 | Hydroxychloroquine in Combination With Sirolimus and Dexamethasone for Treating COVID-19 Patients | Not yet recruiting | Not Applicable | Dec/01/2021 | Sep/01/2022 |
|
|||||
| NCT04341675 | Sirolimus Treatment in Hospitalized Patients With COVID-19 Pneumonia | Recruiting | Phase 2 | Apr/24/2020 | Sep/01/2020 |
|
|||||
