Quinine
A quinidine alkaloid antimalarial.
General information
Quinine is a natural quinidine alkaloid traditionally used for malaria treatment with multiple mechanisms of action (NCIt).
Quinine on DrugBank
Quinine on PubChem
Quinine on Wikipedia
Marketed as
CINKONA; JASOQUIN; QSM; QUALAQUIN; QUININE - ODAN; QUININE SULFATE QUINLUP; QUTIL; SULQUIN
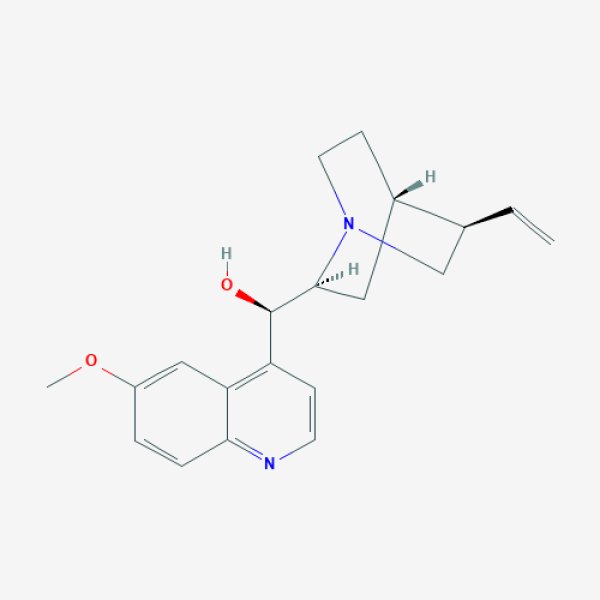
COC1=CC2=C(C=CN=C2C=C1)[C@H]([C@@H]3C[C@@H]4CCN3C[C@@H]4C=C)O
Supporting references
| Link | Tested on | Impact factor | Notes | Publication date |
|---|---|---|---|---|
|
Antimalarial drugs inhibit the replication of SARS-CoV-2: An in vitro evaluation
Small molecule In vitro |
Vero E6 cells; SARS-CoV-2 IHUMI-3 strain | 4.59 | The antimalarial drug inhibited SARS-CoV-2 in vitro at IC50 and IC90 similar to the concentrations reached during oral antimalarial treatment. |
Sep/08/2020 |
|
Quinine Inhibits Infection of Human Cell Lines with SARS-CoV-2
Small molecule In vitro |
Vero B4 cells; Caco-2 cells; Calu-3 cells; TMPRSS2 and/or ACE2-expressing A549 cells | 5.05 | Exerts antiviral activity against SARS-CoV-2 as tested on TMPRSS2-positive human cancer cell lines. It displays lower toxicity compared to chloroquine or hydroxychloroquine. Depending on the cell line used, the observed IC50 was in the range of ca. 3.7 to 50 μM. |
Apr/09/2021 |
AI-suggested references
Clinical trials
| ID | Title | Status | Phase | Start date | Completion date |
|---|---|---|---|---|---|
| NCT04553705 | Omega-3, Nigella Sativa, Indian Costus, Quinine, Anise Seed, Deglycyrrhizinated Licorice, Artemisinin, Febrifugine on Immunity of Patients With (COVID-19) | Recruiting | Phase 2|Phase 3 | Sep/20/2020 | Dec/04/2020 |
|
|||||
