Narlaprevir
A protease inhibitor.
General information
Narlaprevir is a hepatitic C virus (HCV) NS3 protease inhibitor (Tong et al., 2010) approved for chronic HCV infection treatment in Russia (Wikipedia).
Narlaprevir on DrugBank
Narlaprevir on PubChem
Marketed as
ARLANSA
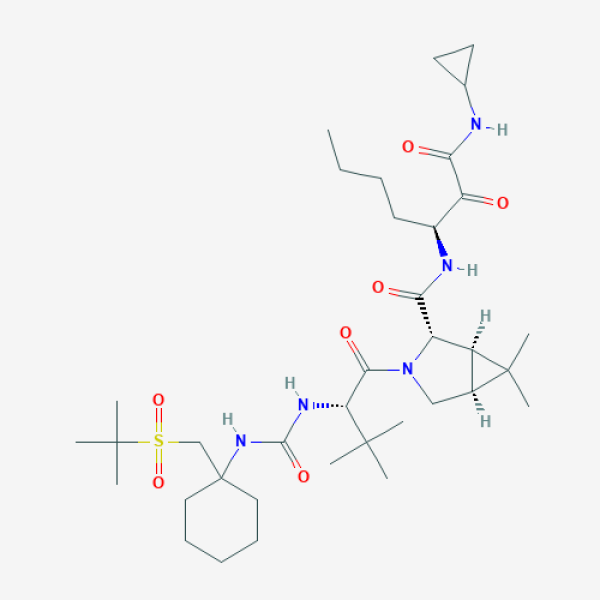
CCCC[C@@H](C(=O)C(=O)NC1CC1)NC(=O)[C@@H]2[C@@H]3[C@@H](C3(C)C)CN2C(=O)[C@H](C(C)(C)C)NC(=O)NC4(CCCCC4)CS(=O)(=O)C(C)(C)C
Supporting references
| Link | Tested on | Impact factor | Notes | Publication date |
|---|---|---|---|---|
|
A drug repurposing screen identifies hepatitis C antivirals as inhibitors of the SARS-CoV2 main protease
3CLpro Enzyme assay In vitro In silico Screening |
in silico; in vitro enzyme assay | 2.74 | Inhibited the SARS-CoV-2 3C-like protease in vitro with IC50 of ca. 1.1 μM. |
Feb/01/2021 |
|
Malleability of the SARS-CoV-2 3CL Mpro Active-Site Cavity Facilitates Binding of Clinical Antivirals
3CLpro Small molecule Enzyme assay In vitro |
in vitro enzyme assay; X-ray crystallography | 4.86 | When co-crystalized with the SARS-CoV-2 3C-like protease (3CLpro), the drug was observed to interact with and structurally modify the enzyme's active site. The drug also inhibited 3CLpro in vitro. |
Nov/08/2020 |
|
Structural basis for the inhibition of the SARS-CoV-2 main protease by the anti-HCV drug narlaprevir
3CLpro Crystallization Biophysical assay Small molecule Enzyme assay In vitro |
in vitro enzyme assay; in vitro biophysical assay; crystallization | 13.49 | The drug binds the SARS-CoV-2 3C-like protease substrate-binding site and inhibits the enzyme with an IC50 of 16.11 μM and no cytotoxicity in Vero cells in concentrations up to 200 μM. |
Feb/04/2021 |
AI-suggested references
| Link | Publication date |
|---|---|
|
Targeting SARS-CoV-2 M3CLpro by HCV NS3/4a Inhibitors: In Silico Modeling and In Vitro Screening
|
Feb/04/2021 |
|
Covalent narlaprevir- and boceprevir-derived hybrid inhibitors of SARS-CoV-2 main protease
|
Apr/27/2022 |
