Montelukast
A leukotriene receptor antagonist.
General information
Montelukast is a leukotriene receptor antagonist used for prophylaxis and treatment of asthma, exercise-induced bronchoconstriction, and seasonal allergic rhinitis (DrugBank).
Montelukast on PubChem
Montelukast on Wikipedia
Marketed as
MONTELUKAST SODIUM (MONTELUKAST SODIUM); SINGULAIR; MONTAIR
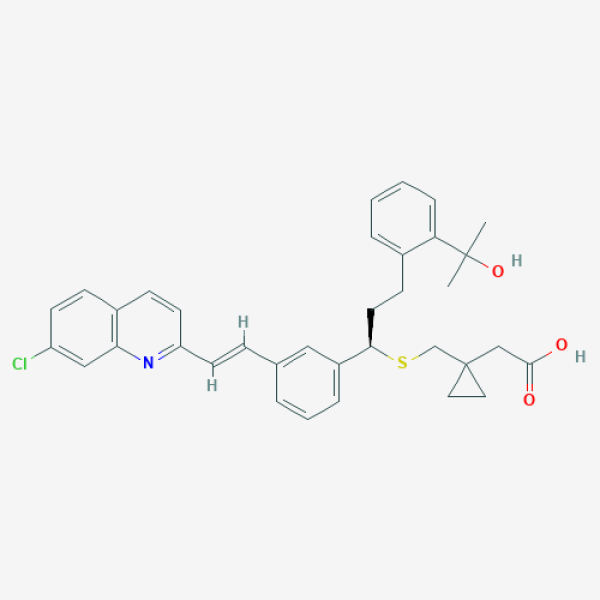
CC(C)(C1=CC=CC=C1CC[C@H](C2=CC=CC(=C2)/C=C/C3=NC4=C(C=CC(=C4)Cl)C=C3)SCC5(CC5)CC(=O)O)O
Supporting references
| Link | Tested on | Impact factor | Notes | Publication date |
|---|---|---|---|---|
|
Discovering drugs to treat coronavirus disease 2019 (COVID-19).
|
in silico | Feb/22/2020 | ||
|
Systemic in Silico Screening in Drug Discovery for Coronavirus Disease (COVID-19) with an Online Interactive Web Server
|
in silico | 4.55 | Predicted to bind a SARS-CoV-2 protein structural feature. |
Aug/11/2020 |
|
Drug repurposing for COVID-19 using machine learning and mechanistic models of signal transduction circuits related to SARS-CoV-2 infection
Protein factor Small molecule Antibody In silico |
in silico (machine learning) | 13.49 | Considered by the authors to be among the most relevant drugs identified in a machine-learning algorithm-based screening of compounds which considers causal protein-protein interactions, known drug targets, and specific signalling circuits in <a href= |
Dec/11/2020 |
|
Screening of drug databank against WT and mutant main protease of SARS-CoV-2: Towards finding potential compound for repurposing against COVID-19
3CLpro Small molecule In silico |
in silico | 2.80 | Predicted to bind SARS-CoV-2 3C-like protease with A191V mutation. |
Feb/23/2021 |
|
Colchicine, aspirin, and montelukast – A case of successful combined pharmacotherapy for adult multisystem inflammatory syndrome in COVID-19
Small molecule Case report |
A patient | Systematic clinical improvement was observed in a patient following the treatment initiation using colchicine, montelukast, and acetylsalicylic acid. Sample size: 1. Dosage: 10 mg daily. |
Nov/30/2020 |
AI-suggested references
Clinical trials
| ID | Title | Status | Phase | Start date | Completion date |
|---|---|---|---|---|---|
| NCT04389411 | The COvid-19 Symptom MOntelukast Trial | Not yet recruiting | Phase 3 | Oct/01/2020 | Dec/01/2021 |
|
|||||
| NCT05094596 | Effect of Montelukast Therapy on Clinical Course, Pulmonary Function, and Mortality in Patients With COVID-19 | Not yet recruiting | Phase 4 | Oct/22/2021 | Jan/22/2022 |
|
|||||
| NCT04714515 | Montelukast - a Treatment Choice for COVID-19 | Completed | Feb/20/2020 | Apr/20/2020 | |
|
|||||
| NCT04718285 | Investigation the Effect of Montelukast in COVID-19 | Not yet recruiting | Phase 2 | Jan/30/2021 | May/30/2021 |
|
|||||
| NCT04695704 | Efficacy of Montelukast in Mild-moderate Respiratory Symptoms in Patients With Long-COVID-19: | Not yet recruiting | Phase 3 | Apr/01/2021 | Dec/31/2022 |
|
|||||
