Lopinavir
Retroviral protease inhibitor.
General information
Lopinavir is a peptidomimetic retroviral protease inhibitor. It is used in combination with ritonavir for the treatment of HIV (NCIt).
Lopinavir/ritonavir therapy used to be one of the antiviral therapies of COVID-19 recommended by China's National Health Commission guidelines. It is NO longer recomended, however (Gui-Qiang et al., 2021).
On July 4, 2020, WHO announced that the lopinavir/ritonavir treatment arm of the Solidarity trial in hospitalized patients with COVID-19 was discontinued.
RECOVERY trial chief investigators stated that based on NO clinical benefit to hospitalized COVID-19 patients treated with lopinavir/ritonavir, further patient recruitment was halted.
A living WHO guideline on drugs for covid-19 (as of March 4, 2022) strongly recommends AGAINST the use of lopinavir/ritonavir combination for the treatment of COVID-19.
Lopinavir on DrugBank
Lopinavir on PubChem
Lopinavir on Wikipedia
Marketed as
ALUVIRAN; KALETRA (fixed-dose with Ritonavir); LOPINAVIR AND RITONAVIR (fixed-dose with Ritonavir)
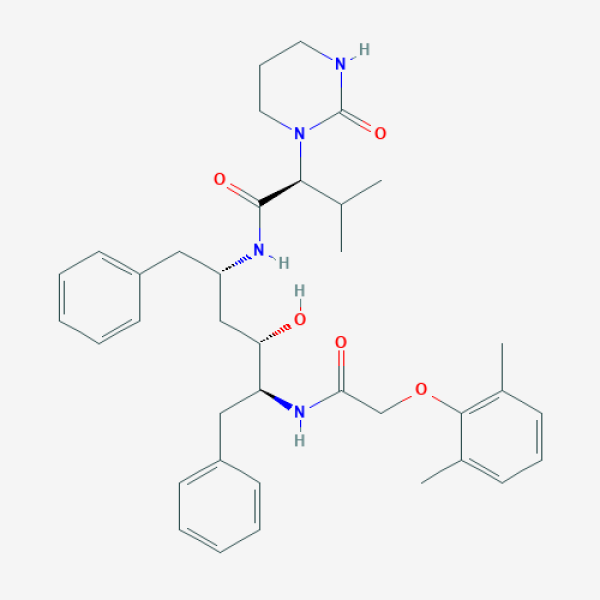
CC1=C(C(=CC=C1)C)OCC(=O)N[C@@H](CC2=CC=CC=C2)[C@H](C[C@H](CC3=CC=CC=C3)NC(=O)[C@H](C(C)C)N4CCCNC4=O)O
Supporting references
| Link | Tested on | Impact factor | Notes | Publication date |
|---|---|---|---|---|
|
Clinical characteristics and therapeutic procedure for four cases with 2019 novel coronavirus pneumonia receiving combined Chinese and Western medicine treatment.
|
Patients | Combination of lopinavir/ritonavir (Kaletra®), arbidol, and Shufeng Jiedu Capsule (SFJDC, a traditional Chinese medicine) |
Mar/16/2020 | |
|
[Potential antiviral therapeutics for 2019 Novel Coronavirus].
|
Similar viruses | In combination with ritonavir or ritonavir and interferon-beta |
Feb/05/2020 | |
|
Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China.
|
Patients | 14.9 % of patients received lopinavir/ritonavir therapy |
Mar/13/2020 | |
|
Epidemiologic Features and Clinical Course of Patients Infected With SARS-CoV-2 in Singapore.
|
Patients | Lopinavir-ritonavir combination was used to treat 5 patients also requiring supplemental oxygen. For 3 of the 5 patients, fever resolved and supplemental oxygen requirement was reduced within 3 days, whereas 2 deteriorated with progressive respiratory failure. Four of the 5 patients treated with lopinavir-ritonavir developed nausea, vomiting, and/or diarrhea, and 3 developed abnormal liver function test results. |
Mar/03/2020 | |
|
Experimental Treatment with Favipiravir for COVID-19: An Open-Label Control Study
|
Patients | Used as lopinavir/ritonavir (Days 1–14: 400 mg/100 mg twice daily) plus IFN-α by aerosol inhalation (5 million U twice daily). Less effective than favipiravir plus IFN-α. |
Mar/18/2020 | |
|
Molecular Modeling Evaluation of the Binding Effect of Ritonavir, Lopinavir and Darunavir to Severe Acute Respiratory Syndrome Coronavirus 2 Proteases
|
in silico | Feb/18/2020 | ||
|
Discovering drugs to treat coronavirus disease 2019 (COVID-19).
|
in silico | Oral, in combination with ritonavir: lopinavir/ritonavir 200 mg/50 mg/capsule, 2 capsules each time, 2 times/day Oral, in combination with ritonavir: lopinavir/ritonavir 200 mg/50 mg/capsule, 2 capsules each time, 2 times/day |
Feb/22/2020 | |
|
A Novel Protein Drug, Novaferon, as the Potential Antiviral Drug for COVID-19
Preprint |
Patients | Better in combination with Novaferon and Ritonavir than only with Ritonavir |
Apr/29/2020 | |
|
Clinical Outcomes and Plasma Concentrations of Baloxavir Marboxil and Favipiravir in COVID-19 Patients: An Exploratory Randomized, Controlled Trial
Small molecule Randomized controlled open trial |
Patients | 3.62 | lopinavir/ritonavir (400mg/100mg, bid, po.) in combination with interferon alpha inhalation (100,000 iu, tid or qid) |
Oct/25/2020 |
|
IDentif.AI: Artificial Intelligence Pinpoints Remdesivir in Combination with Ritonavir and Lopinavir as an Optimal Regimen Against Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)
|
in silico | optimal combination therapy against SARS-CoV-2 was comprised of remdesivir, ritonavir, and lopinavir |
May/08/2020 | |
|
Nelfinavir inhibits replication of severe acute respiratory syndrome coronavirus 2 in vitro
Preprint |
VERO E6 cell cultures | Apr/08/2020 | ||
|
Triple combination of interferon beta-1b, lopinavir–ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial
|
Patients | Combination of lopinavir 400 mg and ritonavir 100 mg every 12 h, ribavirin 400 mg every 12 h, and three doses of 8 million international units of interferon beta-1b on alternate days is better than lopinavir-ritonavir in alleviating symptoms and shortening the duration of viral shedding and hospital stay in patients with mild to moderate COVID-19 |
May/08/2020 | |
|
Why Are Lopinavir and Ritonavir Effective against the Newly Emerged Coronavirus 2019? Atomistic Insights into the Inhibitory Mechanisms
|
in silico | Apr/15/2020 | ||
|
Discovery of Synergistic and Antagonistic Drug Combinations against SARS-CoV-2 In Vitro
Preprint In silico |
VERO E6 cell cultures | synergistic effect in combination with triflupromazine hydrochloride, amodiaquine or arbidol |
Jul/01/2020 | |
|
A retrospective evaluation on the efficacy of Lopinavir/ritonavir and chloroquine to treat non-severe COVID-19 patients
|
Patients | 3.48 | In a fixed-dose combination with Ritonavir. Retrospective study of patients who were aged 18-65 years, were classified as non-severe cases on admission, received treatment at least 72 hours, were admitted into hospital after less than seven days from symptom onset. Sample size 51 patients (+59 control). Dosage 500 mg (LPV/r) twice daily. Endpoints - symptom development/duration; chest CT improvement time; negative conversion of viral nucleic acid. |
Jul/22/2020 |
|
Systemic in Silico Screening in Drug Discovery for Coronavirus Disease (COVID-19) with an Online Interactive Web Server
|
in silico | 4.55 | Predicted to bind a SARS-CoV-2 protein structural feature. |
Aug/11/2020 |
|
Case of the Index Patient Who Caused Tertiary Transmission of COVID-19 Infection in Korea: the Application of Lopinavir/Ritonavir for the Treatment of COVID-19 Infected Pneumonia Monitored by Quantitative RT-PCR.
Case report |
Patients | 1.72 | Combination of lopinavir/ritonavir (Kaletra, AbbVie) |
Feb/17/2020 |
|
Comparative analysis of antiviral efficacy of FDA-approved drugs against SARS-CoV-2 in human lung cells: Nafamostat is the most potent antiviral drug candidate
Preprint |
Calu-3 human airway epithelial cells | higher IC50 value in Calu-3 cells than VERO E6 cells |
May/12/2020 | |
|
In Silico Drug Repurposing for SARS-CoV-2 Main Proteinase and Spike Proteins
Spike protein 3CLpro In silico |
in silico | 4.07 | Predicted to inhibit the SARS-CoV-2 3C-like protease. |
Sep/07/2020 |
|
MCCS: a novel recognition pattern-based method for fast track discovery of anti-SARS-CoV-2 drugs
3CLpro Small molecule In silico |
in silico | 8.99 | Predicted to inhibit the SARS-CoV-2 3C-like protease. |
Oct/20/2020 |
|
Reprofiling of approved drugs against SARS-CoV-2 main protease: an in-silico study
3CLpro Polysaccharide Small molecule In silico |
in silico | 3.22 | Predicted to inhibit the SARS-CoV-2 3C-like protease. |
Nov/12/2020 |
|
Effectiveness of lopinavir/ritonavir on covid-19 related pneumonia in a child with Covid-19 associated Kawasaki disease
Small molecule Case report |
Kawasaki disease child patient | 0.84 | In a fixed-dose with ritonavir. Overall clinical improvement in a 10-year-old patient with COVID-19-associated Kawasaki disease after lopinavir/ritonavir treatment (preceded by unsuccessful hydroxychloroquine and oseltamivir treatment). Dosage: 300 mg daily for 14 days. |
Nov/13/2020 |
|
SARS-CoV-2 entry inhibitors by dual targeting TMPRSS2 and ACE2: An in silico drug repurposing study
ACE2 TMPRSS2 Small molecule In silico |
in silico | 3.26 | Predicted to target both TMPRSS2 and ACE2. |
Feb/02/2021 |
|
Molecular modeling evaluation of the binding effect of five protease inhibitors to COVID-19 main protease
3CLpro Small molecule In silico |
in silico | 1.77 | Predicted to inhibit the SARS-CoV-2 3C-like protease. |
Dec/11/2020 |
|
IDentif.AI: Rapidly optimizing combination therapy design against severe Acute Respiratory Syndrome Coronavirus 2 (SARS‐Cov‐2) with digital drug development
AI Small molecule In vitro In silico |
in silico (AI); AC16 and THLE-2 cells (cytotoxicity); Vero E6 cells | 6.09 | Based on computational assessment and in vitro validation, |
Nov/10/2020 |
|
A Randomized, Double-Blind, Multicenter Clinical Study Comparing the Efficacy and Safety of a Drug Combination of Lopinavir/Ritonavir-Azithromycin, Lopinavir/Ritonavir-Doxycycline, and Azithromycin-Hydroxychloroquine...
Small molecule Randomized controlled double-blind trial Moderate severity Mild severity |
Mild to moderate COVID-19 patients | 2.44 | In combination with |
Feb/09/2021 |
AI-suggested references
Clinical trials
| ID | Title | Status | Phase | Start date | Completion date |
|---|---|---|---|---|---|
| NCT04251871 | Treatment and Prevention of Traditional Chinese Medicines (TCMs) on COVID-19 Infection | Recruiting | Not Applicable | Jan/22/2020 | Jan/22/2021 |
|
|||||
| NCT04350684 | Umifenovir in Hospitalized COVID-19 Patients | Enrolling by invitation | Phase 4 | Apr/15/2020 | Apr/24/2020 |
|
|||||
| NCT02735707 | Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community- Acquired Pneumonia | Recruiting | Phase 3 | Apr/11/2016 | Dec/01/2025 |
|
|||||
| NCT04738045 | Comparison of Remdesivir Versus Lopinavir/ Ritonavir and Remdesivir Combination in COVID-19 Patients | Recruiting | Phase 4 | Nov/01/2020 | Apr/01/2021 |
|
|||||
| NCT04521400 | the Investigation Into Beneficial Effects of High-dose Interferon Beta 1-a, Compared to Low-dose Interferon Beta 1-a in Moderate to Severe Covid-19 | Not yet recruiting | Phase 2 | Aug/20/2020 | Sep/11/2020 |
|
|||||
| NCT04351724 | Austrian CoronaVirus Adaptive Clinical Trial (COVID-19) | Recruiting | Phase 2|Phase 3 | Apr/16/2020 | Mar/31/2022 |
|
|||||
| NCT04376814 | Favipiravir Plus Hydroxychloroquine and Lopinavir/Ritonavir Plus Hydroxychloroquine in COVID-19 | Completed | Not Applicable | Mar/29/2020 | May/25/2020 |
|
|||||
| NCT04466241 | Combination Therapies to Reduce Carriage of SARS-Cov-2 and Improve Outcome of COVID-19 in Ivory Coast: a Phase Randomized IIb Trial | Recruiting | Phase 2|Phase 3 | Nov/27/2020 | Mar/26/2021 |
|
|||||
| NCT04365582 | OUTpatient Treatment of COVID-19 in Patients With Risk Factor for Poor Outcome | Withdrawn | Phase 3 | May/07/2020 | Apr/19/2021 |
|
|||||
| NCT04331470 | Evaluation of Efficacy of Levamisole and Formoterol+Budesonide in Treatment of COVID-19 | Recruiting | Phase 2|Phase 3 | Apr/04/2020 | May/20/2020 |
|
|||||
| NCT04295551 | Multicenter Clinical Study on the Efficacy and Safety of Xiyanping Injection in the Treatment of New Coronavirus Infection Pneumonia (General and Severe) | Unknown status | Not Applicable | Mar/14/2020 | Apr/14/2021 |
|
|||||
| NCT04350671 | Interferon Beta 1a in Hospitalized COVID-19 Patients | Enrolling by invitation | Phase 4 | Apr/15/2020 | Apr/24/2020 |
|
|||||
| NCT04779047 | Comparative Therapeutic Efficacy and Safety of Different Antiviral and Anti Inflammatory Drugs in COVID-19 Patients. | Recruiting | Phase 4 | Oct/01/2020 | Apr/05/2021 |
|
|||||
| NCT04315948 | Trial of Treatments for COVID-19 in Hospitalized Adults | Recruiting | Phase 3 | Mar/22/2020 | Mar/01/2023 |
|
|||||
| NCT04409483 | Evaluation of Additional Treatments for COVID-19: a Randomized Trial in Niger | Withdrawn | Phase 3 | Jun/01/2020 | Dec/31/2020 |
|
|||||
| NCT04328285 | Chemoprophylaxis of SARS-CoV-2 Infection (COVID-19) in Exposed Healthcare Workers | Active, not recruiting | Phase 3 | Apr/14/2020 | Mar/30/2022 |
|
|||||
| NCT04425382 | Darunavir/Cobicistat vs. Lopinavir/Ritonavir in COVID-19 Pneumonia in Qatar | Recruiting | Mar/01/2020 | Sep/01/2020 | |
|
|||||
| NCT04276688 | Lopinavir/ Ritonavir, Ribavirin and IFN-beta Combination for nCoV Treatment | Completed | Phase 2 | Feb/10/2020 | Mar/31/2020 |
|
|||||
| NCT04380818 | Low Dose Anti-inflammatory Radiotherapy for the Treatment of Pneumonia by COVID-19 | Recruiting | Not Applicable | Jun/05/2020 | Nov/01/2021 |
|
|||||
| NCT04275388 | Xiyanping Injection for the Treatment of New Coronavirus Infected Pneumonia | Not yet recruiting | May/15/2020 | Dec/14/2021 | |
|
|||||
| NCT04381936 | Randomised Evaluation of COVID-19 Therapy | Recruiting | Phase 2|Phase 3 | Mar/19/2020 | Nov/01/2032 |
|
|||||
| NCT04386876 | Bioequivalence Study of Lopinavir/Ritonavir 200/50 mg Film Tablet (World Medicine Ilac, Turkey) Under Fasting Conditions | Completed | Phase 1 | Apr/30/2020 | Jun/11/2020 |
|
|||||
| NCT04261907 | Evaluating and Comparing the Safety and Efficiency of ASC09/Ritonavir and Lopinavir/Ritonavir for Novel Coronavirus Infection | Unknown status | Not Applicable | Feb/07/2020 | Jun/30/2020 |
|
|||||
| NCT04455958 | Lopinavir/Ritonavir for the Treatment of COVID-19 Positive Patients With Cancer and Immune Suppression in the Last Year | Withdrawn | Phase 2 | May/01/2021 | Nov/01/2021 |
|
|||||
| NCT04354428 | Treatment for COVID-19 in High-Risk Adult Outpatients | Active, not recruiting | Phase 2|Phase 3 | Apr/16/2020 | Jan/01/2021 |
|
|||||
| NCT04343768 | An Investigation Into Beneficial Effects of Interferon Beta 1a, Compared to Interferon Beta 1b And The Base Therapeutic Regiment in Moderate to Severe COVID-19: A Randomized Clinical Trial | Completed | Phase 2 | Apr/09/2020 | Apr/27/2020 |
|
|||||
| NCT04372628 | Trial of Early Therapies During Non-hospitalized Outpatient Window for COVID-19 | Active, not recruiting | Phase 2 | Jun/01/2020 | Jun/01/2022 |
|
|||||
| NCT04364022 | Efficacy of Pragmatic Same-day COVID-19 Ring Prophylaxis for Adult Individuals Exposed to SARS-CoV-2 in Switzerland | Completed | Phase 3 | Apr/23/2020 | Mar/24/2021 |
|
|||||
| NCT04252885 | The Efficacy of Lopinavir Plus Ritonavir and Arbidol Against Novel Coronavirus Infection | Completed | Phase 4 | Jan/28/2020 | May/31/2020 |
|
|||||
| NCT04286503 | The Clinical Study of Carrimycin on Treatment Patients With COVID-19 | Unknown status | Phase 4 | Feb/23/2020 | Feb/28/2021 |
|
|||||
| NCT04255017 | A Prospective/Retrospective,Randomized Controlled Clinical Study of Antiviral Therapy in the 2019-nCoV Pneumonia | Unknown status | Phase 4 | Feb/01/2020 | Jul/01/2020 |
|
|||||
| NCT04499677 | FLARE: Favipiravir +/- Lopinavir: A RCT of Early Antivirals | Completed | Phase 2 | Sep/24/2020 | Dec/01/2021 |
|
|||||
| NCT04459702 | A Study of Combination Therapies to Treat COVID-19 Infection | Withdrawn | Phase 2 | Jul/01/2020 | Dec/01/2021 |
|
|||||
| NCT04390152 | Safety and Efficacy of Intravenous Wharton's Jelly Derived Mesenchymal Stem Cells in Acute Respiratory Distress Syndrome Due to COVID 19 | Recruiting | Phase 1|Phase 2 | Jan/13/2020 | Apr/01/2022 |
|
|||||
| NCT04321174 | COVID-19 Ring-based Prevention Trial With Lopinavir/Ritonavir | Active, not recruiting | Phase 3 | Apr/17/2020 | Mar/31/2022 |
|
|||||
| NCT05024006 | Public Health Emergency: SOLIDARITY TRIAL Philippines | Completed | Not Applicable | Apr/23/2020 | Apr/17/2021 |
|
|||||
| NCT04307693 | Comparison of Lopinavir/Ritonavir or Hydroxychloroquine in Patients With Mild Coronavirus Disease (COVID-19) | Terminated | Phase 2 | Mar/11/2020 | Apr/30/2020 |
|
|||||
| NCT04403100 | Hydroxychloroquine and Lopinavir/ Ritonavir to Improve the Health of People With COVID-19: "The Hope Coalition - 1" | Recruiting | Phase 3 | Jun/03/2020 | Feb/01/2021 |
|
|||||
| NCT04394182 | Ultra Low Doses of Therapy With Radiation Applicated to COVID-19 | Recruiting | Not Applicable | Apr/21/2020 | Apr/21/2021 |
|
|||||
