Linolenic acid
An omega-3 fatty acid.
General information
Linolenic acid is an essential omega-3 fatty acid with anti-inflammatory properties (NCIt).
Linolenic acid on DrugBank
Linolenic acid on PubChem
Linolenic acid on Wikipedia
Synonyms
α-Linolenic acid; ALA
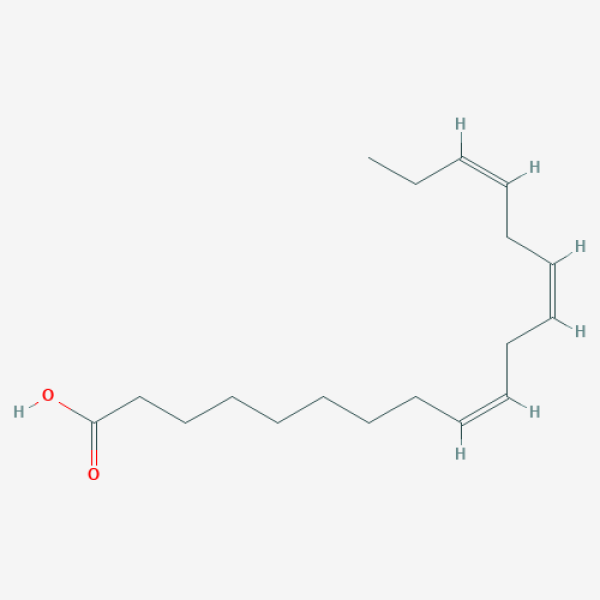
CC/C=C\C/C=C\C/C=C\CCCCCCCC(=O)O
Supporting references
| Link | Tested on | Impact factor | Notes | Publication date |
|---|---|---|---|---|
|
Polyunsaturated ω-3 fatty acids inhibit ACE2-controlled SARS-CoV-2 binding and cellular entry
Spike protein ACE2 TMPRSS2 Cathepsin L Small molecule In vitro |
A549/hACE2 cells; (rVSV) SARS-CoV-2 Spike pseudovirus | 4.00 | The fatty acid blocked SARS-CoV-2 Spike protein pseudovirus entry into cells in vitro. It impeded RBD binding to host ACE2 receptor and reduced the activity (but not protein levels) of TMPRSS2 and cathepsin L proteases. |
Mar/04/2021 |
AI-suggested references
Clinical trials
| ID | Title | Status | Phase | Start date | Completion date |
|---|---|---|---|---|---|
| NCT04604743 | Clinic-based HPV and COVID-19 Vaccine Promoting Intervention for AfAm Adolescents in Alabama | Recruiting | Not Applicable | Apr/20/2021 | Jun/30/2023 |
|
|||||
| NCT04703036 | Glutathione, Oxidative Stress and Mitochondrial Function in COVID-19 | Recruiting | Early Phase 1 | Jan/11/2021 | Dec/31/2023 |
|
|||||
| NCT04405271 | TAF/FTC for Pre-exposure Prophylaxis of COVID-19 in Healthcare Workers (CoviPrep Study) | Not yet recruiting | Phase 3 | Jul/31/2020 | Nov/15/2020 |
|
|||||
