Levamisole
An antiparasitic.
General information
Levamisole is an anthelmintic drug. It is also used as and adjuvant in the cancer treatment due to its immunomodulatory properties (NCIt).
Levamisole on DrugBank
Levamisole on PubChem
Levamisole on Wikipedia
Marketed as
ERGAMISOL; KETRAX
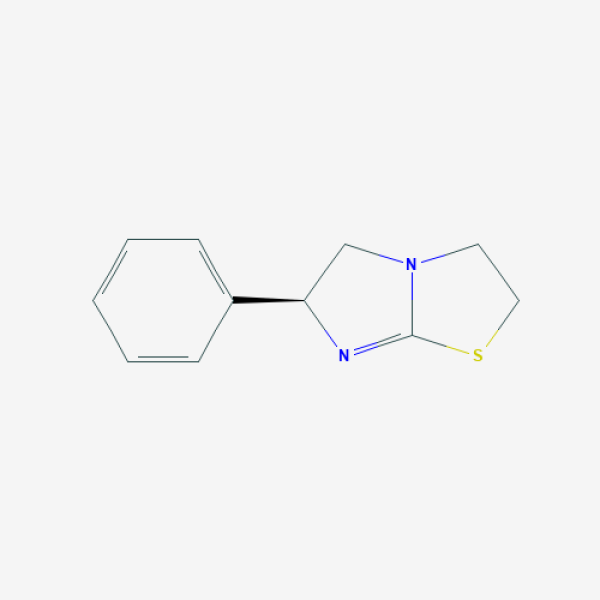
C1CSC2=N[C@H](CN21)C3=CC=CC=C3
Supporting references
| Link | Tested on | Impact factor | Notes | Publication date |
|---|---|---|---|---|
|
Efficacy and safety of Levamisole treatment in clinical presentations of non-hospitalized patients with COVID-19: a double-blind, randomized, controlled trial
Small molecule Randomized controlled double-blind trial |
Outpatients | 2.69 | Statistically significant improvement in cough status (on days 3 and 14) and dyspnoea (on days 7 and 14) were observed in the treatment group compared to control. No significant difference was observed in other clinical parameters or at different time points. The drug is generally safe. Sample size: 25 + 25 placebo. Dosage: 50 mg three times a day for 3 days. |
Mar/24/2021 |
AI-suggested references
Clinical trials
| ID | Title | Status | Phase | Start date | Completion date |
|---|---|---|---|---|---|
| NCT04360122 | Levamisole and Isoprinosine in Immune-prophylaxis of Egyptian Healthcare Workers Facing COVID-19 | Not yet recruiting | Phase 3 | May/20/2020 | Dec/01/2020 |
|
|||||
| NCT04331470 | Evaluation of Efficacy of Levamisole and Formoterol+Budesonide in Treatment of COVID-19 | Recruiting | Phase 2|Phase 3 | Apr/04/2020 | May/20/2020 |
|
|||||
| NCT04383717 | Levamisole and Isoprinosine in the Treatment of COVID19: A Proposed Therapeutic Trial | Not yet recruiting | Phase 3 | May/05/2020 | Oct/30/2020 |
|
|||||
