Lenalidomide
A thalidomide derivative.
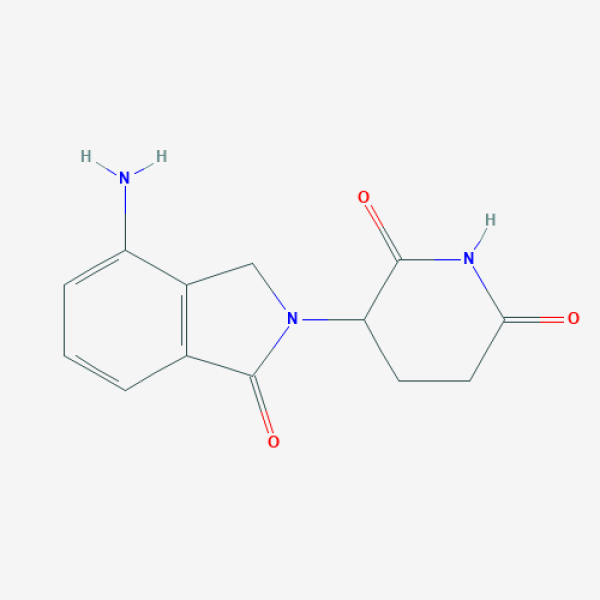
C1CC(=O)NC(=O)C1N2CC3=C(C2=O)C=CC=C3N
Supporting references
| Link | Tested on | Impact factor | Notes | Publication date |
|---|---|---|---|---|
|
Inhibition of SARS-CoV-2 main protease 3CLpro by means of α-ketoamide and pyridone-containing pharmaceuticals using in silico molecular docking
3CLpro Small molecule In silico |
in silico | 2.46 | Predicted to inhibit the SARS-CoV-2 3C-like protease. |
Jul/10/2020 |
AI-suggested references
Clinical trials
| ID | Title | Status | Phase | Start date | Completion date |
|---|---|---|---|---|---|
| NCT04361643 | Low-dose Lenalidomide for Non-severe COVID-19 Treatment Trial | Not yet recruiting | Phase 4 | Oct/27/2020 | Dec/31/2021 |
|
|||||
