Leflunomide
A dihydroorotate dehydrogenase inhibitor prodrug.
General information
Leflunomide is a dihydroorotate dehydrogenase inhibitor prodrug. It has immunosuppressive and anti-inflammatory properties (NCIt). Leflunomide is used for treatment of rheumatoid and psoriatic arthritis (LiverTox).
Leflunomide on DrugBank
Leflunomide on PubChem
Leflunomide on Wikipedia
Marketed as
ARAVA
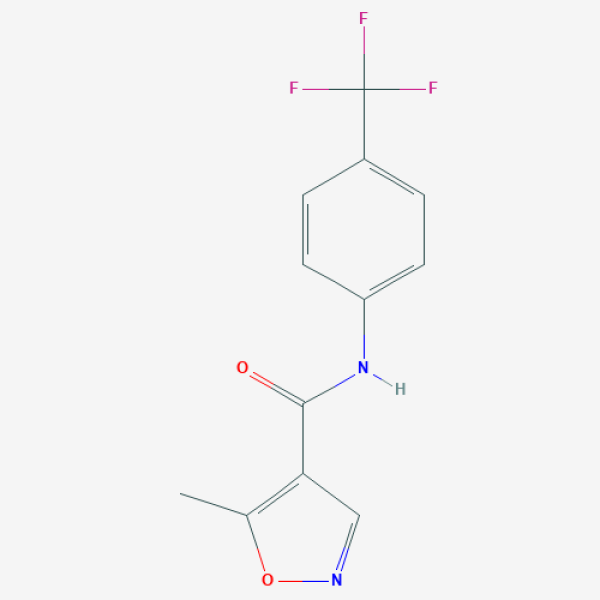
CC1=C(C=NO1)C(=O)NC2=CC=C(C=C2)C(F)(F)F
Supporting references
| Link | Tested on | Impact factor | Notes | Publication date |
|---|---|---|---|---|
|
Multi-omics study revealing tissue-dependent putative mechanisms of SARS-CoV-2 drug targets on viral infections and complex diseases
Preprint |
in silico | May/11/2020 | ||
|
Efficacy and Safety of Leflunomide for Refractory COVID-19: An Open-label Controlled Study
Preprint Non-randomized controlled open trial |
Patients | effective in enhancing SARS-CoV-2 clearance and hospital discharge in refractory COVID-19 patients |
Jun/02/2020 |
AI-suggested references
Clinical trials
| ID | Title | Status | Phase | Start date | Completion date |
|---|---|---|---|---|---|
| NCT04532372 | Leflunomide for the Treatment of Severe COVID-19 in Patients With a Concurrent Malignancy | Recruiting | Phase 1|Phase 2 | Oct/23/2020 | Sep/18/2022 |
|
|||||
| NCT04361214 | Leflunomide in Mild COVID-19 Patients | Recruiting | Phase 1 | May/05/2020 | Feb/01/2021 |
|
|||||
| NCT05007678 | Targeting de Novo Pyrimidine Biosynthesis by Leflunomide for the Treatment of COVID-19 Virus Disease | Active, not recruiting | Phase 3 | Sep/16/2020 | Jul/01/2022 |
|
|||||
