Itraconazole
A triazole antifungal.
General information
Itraconazole is triazole antifungal inhibiting cytochrome P450-dependent ergesterol biosynthetic pathway (NCIt). It is also a Niemann-Pick C1 inhibitor (Zang et al., 2020).
Itraconazole on DrugBank
Itraconazole on PubChem
Itraconazole on Wikipedia
Synonyms
Sporanox; ICZ
Marketed as
ITRACONAZOLE; ITRIZOLE; ONMEL; ORICONAZOLE; SPORAL; SPORANOX; TOLSURA
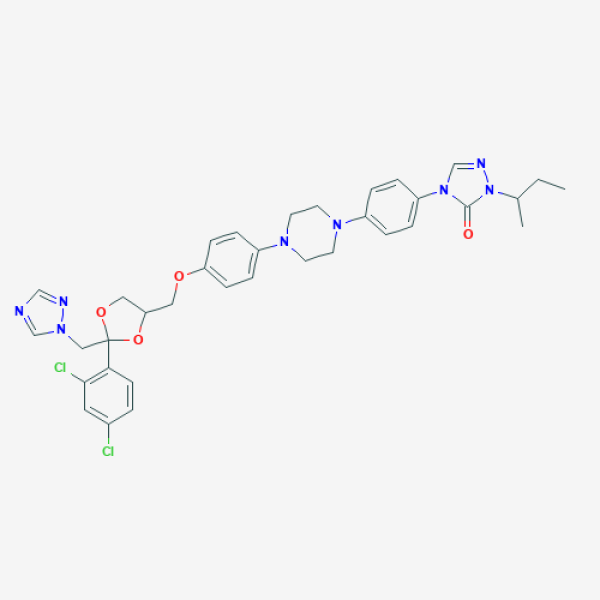
CCC(C)N1C(=O)N(C=N1)C2=CC=C(C=C2)N3CCN(CC3)C4=CC=C(C=C4)OCC5COC(O5)(CN6C=NC=N6)C7=C(C=C(C=C7)Cl)Cl
Supporting references
| Link | Tested on | Impact factor | Notes | Publication date |
|---|---|---|---|---|
|
Identification of an Antiviral Compound from the Pandemic Response Box that Efficiently Inhibits SARS-CoV-2 Infection In Vitro
Small molecule In vitro |
Vero E6 cells; Calu-3 cells | 4.15 | less potent than Remdesivir |
Nov/26/2020 |
|
Cholesterol 25-hydroxylase suppresses SARS-CoV-2 replication by blocking membrane fusion
Small molecule In vitro |
Vero E6 cells; HEK293 cells; HEK293-hACE2 cells; MA104 cells; primary human intestinal epithelial cells; cardiomyocytes | 9.41 | Inhibited chimeric SARS-CoV-2 virus replication in vitro. It also experimentally reduced S-protein-mediated cell-to-cell fusion. |
Nov/25/2020 |
|
In vitro activity of itraconazole against SARS‐CoV‐2
Small molecule In vitro |
Caco‐2 cells; VeroE6‐eGFP cells; SARS-CoV-2 strain hCoV-19/Germany/FrankfurtFFM1/2020; SARS-CoV-2 strain BetaCov/Belgium/GHB-03021/2020 | 2.02 | Inhibited SARS-CoV-2 infection in Caco-2 cells in a dose-dependent manner with an EC50 of 1.5 μM and 2.3 μM in two different assays. At 6.25 μM, the compound reduced SARS-CoV-2 RNA loads in Caco-2 cells by ca. 2 orders of magnitude (ca. 10 times lower reduction compared to remdesivir, however). At 1 μM, the compound reduced SARS-CoV-2 RNA loads in VeroE6-eGFP cells by a factor of ca. 10. |
Mar/05/2021 |
AI-suggested references
Clinical trials
| ID | Title | Status | Phase | Start date | Completion date |
|---|---|---|---|---|---|
| NCT04962022 | Drug-Drug Interaction Study Assessing Effect of Itraconazole on PF-07321332/Ritonavir in Healthy Participants | Completed | Phase 1 | Jul/20/2021 | Sep/30/2021 |
|
|||||
| NCT04577378 | Efficacy and Safety of Drug Combination Therapy of Isotretinoin and Some Antifungal Drugs as A Potential Aerosol Therapy for COVID-19 : An Innovative Therapeutic Approach COVID-19 | Not yet recruiting | Phase 2 | Oct/20/2020 | Nov/20/2020 |
|
|||||
