Isavuconazonium
A triazole antifungal.
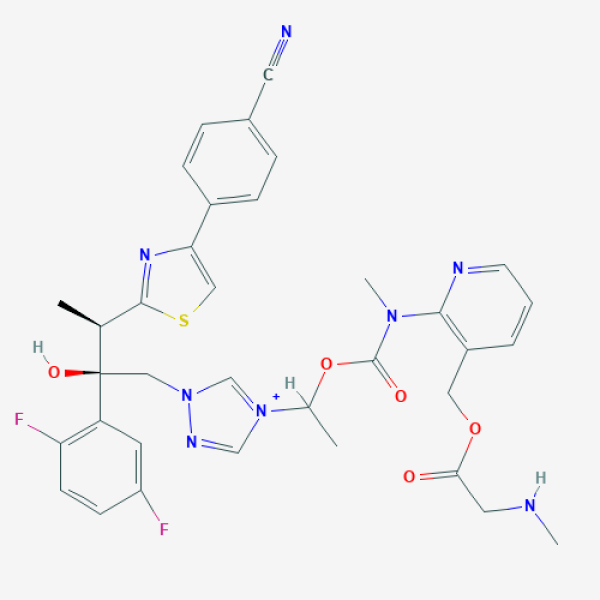
C[C@@H](C1=NC(=CS1)C2=CC=C(C=C2)C#N)[C@](CN3C=[N+](C=N3)C(C)OC(=O)N(C)C4=C(C=CC=N4)COC(=O)CNC)(C5=C(C=CC(=C5)F)F)O
Supporting references
| Link | Tested on | Impact factor | Notes | Publication date |
|---|---|---|---|---|
|
Targeting the SARS-CoV-2 main protease using FDA-approved Isavuconazonium, a P2–P3 α-ketoamide derivative and Pentagastrin: An in-silico drug discovery approach
3CLpro Small molecule In silico |
in silico | 2.08 | Predicted to bind the SARS-CoV-2 3C-like protease. |
Sep/02/2020 |
AI-suggested references
| Link | Publication date |
|---|---|
|
Targeting the SARS-CoV-2 main protease using FDA-approved Isavuconazonium, a P2-P3 alpha-ketoamide derivative and Pentagastrin: An in-silico drug discovery approach.
|
Sep/02/2020 |
Clinical trials
| ID | Title | Status | Phase | Start date | Completion date |
|---|---|---|---|---|---|
| NCT04707703 | Isavuconazole for the Prevention of COVID-19-associated Pulmonary Aspergillosis | Recruiting | Phase 3 | Mar/16/2021 | Mar/01/2022 |
|
|||||
