Ibuprofen
A nonsteroidal anti-inflammatory drug.
General information
Ibuprofen on PubChem
Marketed as
Sold under multiple prescription and over the counter product brands.
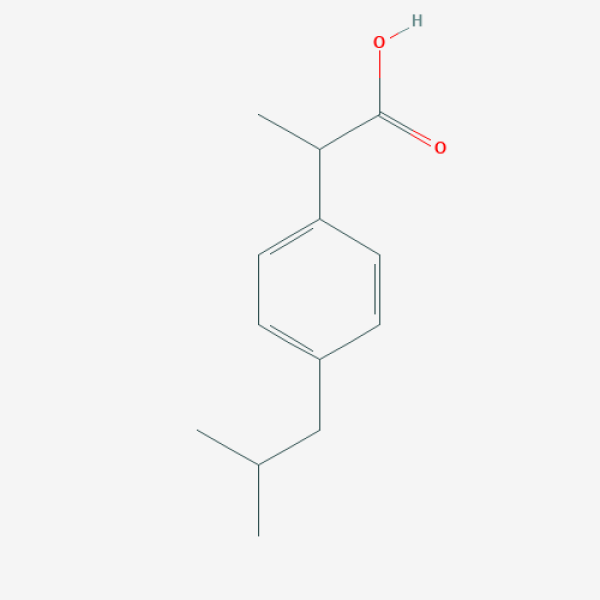
CC(C)CC1=CC=C(C=C1)C(C)C(=O)O
Supporting references
| Link | Tested on | Impact factor | Notes | Publication date |
|---|---|---|---|---|
|
An in silico analysis of Ibuprofen enantiomers in high concentrations of sodium chloride with SARS-CoV-2 main protease
3CLpro Small molecule In silico |
in silico | 3.22 | Both enantiomers (R(−) and (S(+)) were predicted to bind the SARS-CoV-2 3C-like protease. |
Jan/18/2021 |
AI-suggested references
Clinical trials
| ID | Title | Status | Phase | Start date | Completion date |
|---|---|---|---|---|---|
| NCT04334629 | LIBERATE Trial in COVID-19 | Recruiting | Phase 4 | May/26/2020 | Sep/25/2021 |
|
|||||
| NCT04382768 | Inhaled Ibuprofen to Treat COVID-19 | Recruiting | Not Applicable | May/01/2020 | Jan/01/2021 |
|
|||||
| NCT04383899 | Role of Ibuprofen and Other Medicines on Severity of Coronavirus Disease 2019 | Withdrawn | Sep/30/2020 | Nov/30/2020 | |
|
|||||
