|
NCT04389359
|
PROphylaxis for paTiEnts at Risk of COVID-19 infecTion |
Withdrawn |
Phase 2|Phase 3 |
Sep/01/2020 |
Aug/01/2025 |
- Alternative id - A095590
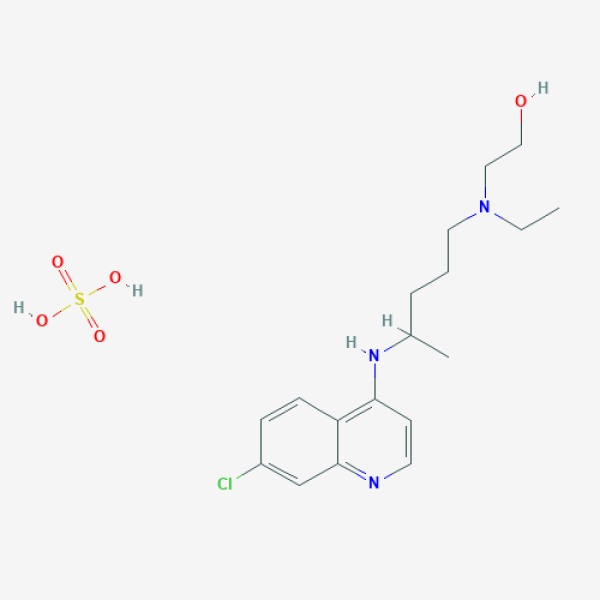
- Interventions - Drug: Hydroxychloroquine Sulfate 200 MG
- Study type - Interventional
- Study results - No Results Available
- Locations -
- Study designs - Allocation: Randomized|Intervention Model: Parallel Assignment|Masking: None (Open Label)|Primary Purpose: Prevention
- Enrollment - 0
- Age - 18 Years and older (Adult, Older Adult)
- Outcome measures - Time to confirmed diagnosis of COVID-19|All-cause mortality|Severity of COVID-19 disease|Incidence of COVID-19 complications
|
|
NCT04371926
|
Prophylactic Benefit of Hydroxychloroquine in COVID-19 Cases With Mild to Moderate Symptoms and in Healthcare Workers With High Exposure Risk |
Withdrawn |
Not Applicable |
Jun/01/2020 |
Jul/01/2021 |
- Alternative id - TCAI_PREVENT
- Interventions - Drug: Hydroxychloroquine Sulfate
- Study type - Interventional
- Study results - No Results Available
- Locations -
- Study designs - Allocation: Randomized|Intervention Model: Parallel Assignment|Masking: Single (Investigator)|Primary Purpose: Prevention
- Enrollment - 0
- Age - 18 Months to 85 Years (Child, Adult, Older Adult)
- Outcome measures - Time to reach normal body temperature|Development of COVID-19 symptoms during HCQ preventive therapy in staff|COVID-19 test result at follow-up in patients|Worsening of symptoms in COVID-19 patients
|
|
NCT04329572
|
Efficacy and Safety of Hydroxychloroquine and Azithromycin for the Treatment of Hospitalized Patients With Moderate to Severe COVID-19 |
Suspended |
Early Phase 1 |
Apr/23/2020 |
Jun/30/2020 |
- Alternative id - HIAPRE0320OR
- Interventions - Drug: Hydroxychloroquine Sulfate|Drug: Azithromycin Tablets
- Study type - Interventional
- Study results - No Results Available
- Locations - Prevent Senior Private Operadora de Saúde LTDA., São Paulo, Brazil
- Study designs - Allocation: N/A|Intervention Model: Single Group Assignment|Masking: None (Open Label)|Primary Purpose: Treatment
- Enrollment - 400
- Age - 18 Years and older (Adult, Older Adult)
- Outcome measures - Evolution of acute respiratory syndrome, oxygen saturation hemodynamic stability|Viral load|Change in Clinical Condition|Evolution of Acute Respiratory Syndrome|Hospital discharge|Rate of mortality within 28-days
|
|
NCT04354441
|
Effect of Hydroxychloroquine in COVID-19 Positive Pregnant Women |
Withdrawn |
Phase 2 |
May/01/2020 |
May/01/2020 |
- Alternative id - 1, March 30, 2020
- Interventions - Drug: hydroxychloroquine sulfate 200 MG|Drug: Placebo oral tablet
- Study type - Interventional
- Study results - No Results Available
- Locations -
- Study designs - Allocation: Randomized|Intervention Model: Parallel Assignment|Masking: Quadruple (Participant, Care Provider, Investigator, Outcomes Assessor)|Primary Purpose: Treatment
- Enrollment - 0
- Age - 18 Years to 50 Years (Adult)
- Outcome measures - COVID-19-related hospital admissions|Symptoms related to COVID-19 infection|Adverse Events|Maternal outcomes|Newborn outcomes
|
|
NCT04384380
|
Efficacy and Tolerability of Hydroxychloroquine in Adult Patients With COVID-19 |
Completed |
Not Applicable |
Apr/01/2020 |
May/31/2020 |
- Alternative id - TYGH109014
- Interventions - Drug: Hydroxychloroquine Sulfate 200 MG [Plaquenil]
- Study type - Interventional
- Study results - No Results Available
- Locations - Taoyuan General Hospital, Ministry of Health and Welfare, Taoyuan City, Taiwan
- Study designs - Allocation: Randomized|Intervention Model: Parallel Assignment|Masking: None (Open Label)|Primary Purpose: Treatment
- Enrollment - 33
- Age - 20 Years to 79 Years (Adult, Older Adult)
- Outcome measures - Time to negatively RT-PCR|Virologic assessment|Number of participants with treatment-related adverse events as assessed by CTCAE v4.0
|
|
NCT04380818
|
Low Dose Anti-inflammatory Radiotherapy for the Treatment of Pneumonia by COVID-19 |
Recruiting |
Not Applicable |
Jun/05/2020 |
Nov/01/2021 |
- Alternative id - IPACOVID
- Interventions - Radiation: Low-dose radiotherapy|Drug: Hydroxychloroquine Sulfate|Drug: Ritonavir/lopinavir|Drug: Tocilizumab Injection [Actemra]|Drug: Azithromycin|Drug: Corticosteroid|Drug: Low molecular weight heparin|Device: Oxygen supply
- Study type - Interventional
- Study results - No Results Available
- Locations - Hospital Sant Joan de Reus, Reus, Tarragona, Spain|Hospital Del Mar, Barcelona, Spain|Hospital Universitario Madrid Sanchinarro, Madrid, Spain
- Study designs - Allocation: Non-Randomized|Intervention Model: Parallel Assignment|Masking: None (Open Label)|Primary Purpose: Supportive Care
- Enrollment - 106
- Age - 18 Years to 99 Years (Adult, Older Adult)
- Outcome measures - Efficacy of low-dose pulmonary irradiation assessed by change in PAFI O2 by 20%|Number of participants with treatment-related adverse events as assessed by CTCAE v5.0|Change of the radiological image|Overall mortality|Measure of pro-inflammatory interleukins|Measure of trasforming growth factor (TGF-b)|Measure of tumor necrosis factor alpha (TNF-a)|Determining overexpression of pro-inflammatory selectin|Determining cell adhesion molecules (CAMs)|Measure of marker of oxidative stress PON-1
|
|
NCT04359537
|
Efficacy of Various Doses of Hydroxychloroquine in Pre-Exposure Prophylaxis for COVID 19 |
Recruiting |
Phase 2 |
May/01/2020 |
Sep/25/2020 |
- Alternative id - F.1-1/2015/ERB/SZABMU/549
- Interventions - Drug: Hydroxychloroquine Sulfate 200 MG|Other: Placebo
- Study type - Interventional
- Study results - No Results Available
- Locations - Shaheed Zulfiaqar Ali Bhutto Medical University, Islamabad, Federal Capital, Pakistan
- Study designs - Allocation: Randomized|Intervention Model: Parallel Assignment|Masking: Single (Participant)|Primary Purpose: Prevention
- Enrollment - 200
- Age - 18 Years to 60 Years (Adult)
- Outcome measures - COVID-19-free survival in experimental arms compared to placebo|Incidence of confirmed SARS-COV-2 detection|Incidence of possible COVID-19 symptoms|Incidence of all-cause study medicine discontinuation|Ordinal Scale of COVID-19 Disease maximum severity if COVID-19 diagnosed at study end|Incidence of Hospitalization for COVID-19 or death|Incidence of study medication-related adverse events
|
|
NCT04326725
|
Proflaxis Using Hydroxychloroquine Plus Vitamins-Zinc During COVID-19 Pandemia |
Active, not recruiting |
|
Mar/20/2020 |
Sep/01/2020 |
- Alternative id - 2020-2/1
- Interventions - Drug: Plaquenil 200Mg Tablet
- Study type - Observational
- Study results - No Results Available
- Locations - Istinye University Medical School, Istanbul, Turkey
- Study designs - Observational Model: Case-Control|Time Perspective: Prospective
- Enrollment - 80
- Age - 20 Years to 90 Years (Adult, Older Adult)
- Outcome measures - Protection against COVID-19
|
|
NCT04346667
|
Post-Exposure Prophylaxis for Asymptomatic SARS-CoV-2 COVID-19 Patients With choloroquinE Compounds |
Terminated |
Phase 4 |
Apr/14/2020 |
Aug/30/2020 |
- Alternative id - NBC-COVID19-02
- Interventions - Drug: Hydroxychloroquine Sulfate Regular dose|Drug: Hydroxychloroquine Sulfate Loading Dose|Drug: Chloroquine|Drug: Placebo
- Study type - Interventional
- Study results - No Results Available
- Locations - Expo Covid Center, Lahore, Punjab, Pakistan|Mayo Hospital, Lahore, Punjab, Pakistan|Pakistan Kidney and Liver Institute, Lahore, Punjab, Pakistan
- Study designs - Allocation: Randomized|Intervention Model: Parallel Assignment|Masking: Double (Participant, Outcomes Assessor)|Primary Purpose: Treatment
- Enrollment - 125
- Age - 20 Years to 50 Years (Adult)
- Outcome measures - RT-PCR negative status|Progression of symptoms|Development of Symptoms|Adverse events
|
|
NCT04340349
|
Low-dose Hydroxychloroquine and Bromhexine: a Novel Regimen for COVID-19 Prophylaxis in Healthcare Professionals |
Enrolling by invitation |
Early Phase 1 |
Feb/01/2021 |
Jun/30/2021 |
- Alternative id - 25/20
- Interventions - Drug: Hydroxychloroquine Sulfate|Drug: Bromhexine 8 MG
- Study type - Interventional
- Study results - No Results Available
- Locations - National Institute of Rehabilitation, Luis Guillermo Ibarra Ibarra, Mexico City, Cdmx, Mexico
- Study designs - Allocation: Randomized|Intervention Model: Parallel Assignment|Masking: Triple (Participant, Care Provider, Investigator)|Primary Purpose: Prevention
- Enrollment - 214
- Age - 18 Years to 59 Years (Adult)
- Outcome measures - Quantification of the expression of mRNA SARS-CoV-2 and presence or absence of antibodies anti-SARS-CoV-2
|
|
NCT04361461
|
Use of Hydroxychloroquine Alone or Associated for Inpatients With SARS-CoV2 Virus (COVID-19) |
Withdrawn |
Phase 3 |
Apr/30/2020 |
Nov/04/2020 |
- Alternative id - APS000/2020
- Interventions - Drug: Hydroxychloroquine Sulfate|Drug: Hydroxychloroquine Sulfate + Azythromycin
- Study type - Interventional
- Study results - No Results Available
- Locations - Apsen Farmacêutica S.A., São Paulo, Brazil
- Study designs - Allocation: Randomized|Intervention Model: Parallel Assignment|Masking: None (Open Label)|Primary Purpose: Treatment
- Enrollment - 0
- Age - 18 Years and older (Adult, Older Adult)
- Outcome measures - Individual response rate|All-cause mortality|Duration of mechanical ventilation|Proportion of patients which needed mechanical ventilation during study|World Health Organization (WHO) Ordinal scale|Duration of hospitalization|Rates of drug discontinuation
|
|
NCT04446104
|
A Preventive Treatment for Migrant Workers at High-risk of COVID-19 |
Completed |
Phase 3 |
May/13/2020 |
Aug/31/2020 |
- Alternative id - 2020/00561
- Interventions - Drug: Hydroxychloroquine Sulfate Tablets|Drug: Ivermectin 3mg Tab|Drug: Zinc|Drug: Povidone-Iodine|Dietary Supplement: Vitamin C
- Study type - Interventional
- Study results - No Results Available
- Locations - Tuas South Dormitory, Singapore, Singapore
- Study designs - Allocation: Randomized|Intervention Model: Parallel Assignment|Masking: None (Open Label)|Primary Purpose: Prevention
- Enrollment - 4257
- Age - 21 Years to 60 Years (Adult)
- Outcome measures - Laboratory-confirmed COVID-19 in treatment arms (hydroxychloroquine, ivermectin, zinc and povidone iodine)|Acute respiratory illness in treatment arms (hydroxychloroquine, ivermectin, zinc and povidone iodine)|Febrile respiratory illness in treatment arms (hydroxychloroquine, ivermectin, zinc and povidone iodine)|Rate of hospitalization for COVID-19 and non-COVID-19 related indications in treatment arms (hydroxychloroquine, ivermectin, zinc and povidone iodine)|Rate of oxygen supplementation and mechanical ventilation in treatment arms (hydroxychloroquine, ivermectin, zinc and povidone iodine)|Duration of oxygen supplementation and mechanical ventilation in treatment arms (hydroxychloroquine, ivermectin, zinc and povidone iodine)|Length of hospital stay in treatment arms (hydroxychloroquine, ivermectin, zinc and povidone iodine)|Rate of laboratory-confirmed COVID-19 in treatment arms (hydroxychloroquine, ivermectin, zinc and povidone iodine)|Adverse events and serious adverse events in control arm (Vitamin C)|Drug discontinuation due to adverse events in control arm (Vitamin C)
|
|
NCT04342156
|
Safety And Efficacy Of Hydroxychloroquine For At Risk Population (SHARP) Against COVID-19 |
Withdrawn |
Phase 3 |
Apr/01/2020 |
Oct/01/2020 |
- Alternative id - 2020/00402
- Interventions - Drug: Hydroxychloroquine Sulfate 200 milligram (mg) Tab
- Study type - Interventional
- Study results - No Results Available
- Locations -
- Study designs - Allocation: Randomized|Intervention Model: Parallel Assignment|Masking: None (Open Label)|Primary Purpose: Prevention
- Enrollment - 0
- Age - 18 Years to 80 Years (Adult, Older Adult)
- Outcome measures - positive serology or reverse transcriptase (RT-PCR) for COVID-19 up until day 28.|Positive serology at day 28.|Symptoms of COVID-19.
|
|
NCT04374552
|
Asymptomatic COVID-19 Trial |
Withdrawn |
Phase 2 |
May/05/2020 |
Apr/01/2021 |
- Alternative id - Pro2020000872
- Interventions - Drug: Hydroxychloroquine sulfate &Azithromycin|Drug: Placebo
- Study type - Interventional
- Study results - No Results Available
- Locations - Robert Wood Johnson University Hospital, New Brunswick, New Jersey, United States
- Study designs - Allocation: Randomized|Intervention Model: Parallel Assignment|Masking: Quadruple (Participant, Care Provider, Investigator, Outcomes Assessor)|Primary Purpose: Treatment
- Enrollment - 0
- Age - 20 Years and older (Adult, Older Adult)
- Outcome measures - The primary outcome is the rate of decline in viral load over the 10 days after randomization
|
|
NCT04354428
|
Treatment for COVID-19 in High-Risk Adult Outpatients |
Active, not recruiting |
Phase 2|Phase 3 |
Apr/16/2020 |
Jan/01/2021 |
- Alternative id - STUDY00009878|INV-017062
- Interventions - Drug: Ascorbic Acid|Drug: Hydroxychloroquine Sulfate|Drug: Azithromycin|Drug: Folic Acid|Drug: Lopinavir 200 MG / Ritonavir 50 MG [Kaletra]
- Study type - Interventional
- Study results - No Results Available
- Locations - Ruth M. Rothstein CORE Center - Cook County Health, Chicago, Illinois, United States|Tulane University, New Orleans, Louisiana, United States|Boston University, Boston, Massachusetts, United States|SUNY Upstate Medical University, Syracuse, New York, United States|University of Washington Coordinating Center, Seattle, Washington, United States|UW Virology Research Clinic, Seattle, Washington, United States
- Study designs - Allocation: Randomized|Intervention Model: Parallel Assignment|Masking: Double (Participant, Investigator)|Primary Purpose: Treatment
- Enrollment - 300
- Age - 18 Years to 80 Years (Adult, Older Adult)
- Outcome measures - Lower respiratory tract infection (LRTI) rates|Incidence of hospitalization or mortality|Change in upper respiratory viral shedding|COVID-19 symptom resolution rates [Lopinavir-ritonavir arm only]|Rate of participant-reported adverse events|COVID-19-related hospitalization days|Rate of disease severity|Viral shedding rates|Individual lopinavir-ritonavir concentration profiles and exposure estimates [Lopinavir-ritonavir arm only]
|
|
NCT04345653
|
Hydroxychloroquine as Chemoprevention for COVID-19 for High Risk Healthcare Workers |
Completed |
Phase 2 |
Apr/14/2020 |
May/10/2021 |
- Alternative id - Pro2020-0356
- Interventions - Drug: Hydroxychloroquine Sulfate (HCQ)
- Study type - Interventional
- Study results - Has Results
- Locations - Hackensack Meridian Health - JFK Medical Center, Edison, New Jersey, United States
- Study designs - Allocation: N/A|Intervention Model: Single Group Assignment|Masking: None (Open Label)|Primary Purpose: Prevention
- Enrollment - 48
- Age - 18 Years to 99 Years (Adult, Older Adult)
- Outcome measures - Recruitment Feasibility|Resource Utilization|Safety as Reflected on the Number and Severity of Adverse Events and Serious Adverse Events|Early Feasibility as Reflected on the Number of Participants Contracting COVID-19 (10% or Less) in Comparison to the Expected 30% as Per CDC.
|
|
NCT04348474
|
Efficacy and Safety of Hydroxychloroquine and Azithromycin for the Treatment of Ambulatory Patients With Mild COVID-19 |
Suspended |
Early Phase 1 |
Apr/20/2020 |
Jul/31/2020 |
- Alternative id - HIAPRE0420OR
- Interventions - Drug: Hydroxychloroquine Sulfate|Drug: Azithromycin Tablets
- Study type - Interventional
- Study results - No Results Available
- Locations - Prevent Senior Private Operadora de Saúde LTDA., São Paulo, Brazil
- Study designs - Allocation: N/A|Intervention Model: Single Group Assignment|Masking: None (Open Label)|Primary Purpose: Treatment
- Enrollment - 200
- Age - 18 Years and older (Adult, Older Adult)
- Outcome measures - Change in Clinical Condition|Hospitalization|Rate of mortality within 28-days|Change in Clinical Condition related to comorbidity
|
|
NCT04329923
|
The PATCH Trial (Prevention And Treatment of COVID-19 With Hydroxychloroquine) |
Terminated |
Phase 2 |
Apr/09/2020 |
Nov/13/2020 |
- Alternative id - 842838
- Interventions - Drug: Hydroxychloroquine Sulfate 400 mg twice a day|Drug: Hydroxychloroquine Sulfate 600 mg twice a day|Drug: Hydroxychloroquine Sulfate 600 mg once a day|Drug: Placebo oral tablet
- Study type - Interventional
- Study results - Has Results
- Locations - University of Pennsylvania, Philadelphia, Pennsylvania, United States
- Study designs - Allocation: Randomized|Intervention Model: Parallel Assignment|Masking: Triple (Participant, Care Provider, Investigator)|Primary Purpose: Treatment
- Enrollment - 173
- Age - 18 Years and older (Adult, Older Adult)
- Outcome measures - Time to Release From Quarantine Time|Time to Hospital Discharge|Number of Health Care Workers Who Developed SARS-COV-2 Infection|Rate of Housemate Infection|Rate of Hospitalization
|
|
NCT04336332
|
Randomized Comparison of Combination Azithromycin and Hydroxychloroquine vs. Hydroxychloroquine Alone for the Treatment of Confirmed COVID-19 |
Active, not recruiting |
Phase 2 |
Apr/01/2020 |
Jun/30/2022 |
- Alternative id - Pro2020000712|002011
- Interventions - Combination Product: Hydroxychloroquine Sulfate + Azithromycin|Drug: Hydroxychloroquine Sulfate
- Study type - Interventional
- Study results - No Results Available
- Locations - Saint Barnabas Medical Center, Livingston, New Jersey, United States|Robert Wood Johnson University Hopsital, New Brunswick, New Jersey, United States|Rutgers Cancer Institute of New Jersey, New Brunswick, New Jersey, United States|The University Hospital, Newark, New Jersey, United States
- Study designs - Allocation: Randomized|Intervention Model: Parallel Assignment|Masking: None (Open Label)|Primary Purpose: Treatment
- Enrollment - 160
- Age - 18 Years and older (Adult, Older Adult)
- Outcome measures - Changes in patients viral load|Second evaluation of changes in patients viral load|Symptom questionnaire|Fever assessment|Vital Signs - Body Temperature|Discharge|Recovery|Assessment of agent toxicity|Oropharynx swab sample collections|Blood Sample collections|Viral shedding assessment - nasopharyngeal secretions|Viral shedding assessment - serology|Cytokines in blood
|
|
NCT04341727
|
Hydroxychloroquine,Hydroxychloroquine,Azithromycin in the Treatment of SARS CoV-2 Infection |
Terminated |
Phase 3 |
Apr/04/2020 |
Apr/01/2021 |
- Alternative id - 202003188
- Interventions - Drug: Hydroxychloroquine Sulfate|Drug: Azithromycin|Drug: Chloroquine Sulfate
- Study type - Interventional
- Study results - No Results Available
- Locations - Washington University School of Medicine Infectious Disease Clinical Research Unit, Saint Louis, Missouri, United States|Washington University School of Medicine, Saint Louis, Missouri, United States
- Study designs - Allocation: Randomized|Intervention Model: Parallel Assignment|Masking: None (Open Label)|Primary Purpose: Treatment
- Enrollment - 31
- Age - 18 Years and older (Adult, Older Adult)
- Outcome measures - Hours to recovery|Time fever resolution
|
|
NCT04342221
|
Hydroxychloroquine for COVID-19 |
Terminated |
Phase 3 |
Mar/29/2020 |
Feb/26/2021 |
- Alternative id - COV-HCQ|2020-001224-33
- Interventions - Drug: Hydroxychloroquine Sulfate|Drug: Placebo
- Study type - Interventional
- Study results - No Results Available
- Locations - Zollernalb Klinikum Balingen, Balingen, Germany|Klinikum Darmstadt, Darmstadt, Germany|Universitätsklinikum Hamburg-Eppendorf, Hamburg, Germany|Johannes Wesling Klinikum Minden, Minden, Germany|Klinikum am Steinenberg, Reutlingen, Germany|RoMed Klinikum Rosenheim, Rosenheim, Germany|Robert-Bosch-Krankenhaus, Stuttgart, Germany|Institute for Tropical Medicine, Tübingen, Germany
- Study designs - Allocation: Randomized|Intervention Model: Parallel Assignment|Masking: Quadruple (Participant, Care Provider, Investigator, Outcomes Assessor)|Primary Purpose: Treatment
- Enrollment - 30
- Age - 18 Years to 99 Years (Adult, Older Adult)
- Outcome measures - Effect of HCQ on in vivo viral clearance
|
|
NCT04307693
|
Comparison of Lopinavir/Ritonavir or Hydroxychloroquine in Patients With Mild Coronavirus Disease (COVID-19) |
Terminated |
Phase 2 |
Mar/11/2020 |
Apr/30/2020 |
- Alternative id - S2020-0472-0001
- Interventions - Drug: Lopinavir/ritonavir|Drug: Hydroxychloroquine sulfate
- Study type - Interventional
- Study results - No Results Available
- Locations - Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea, Republic of
- Study designs - Allocation: Randomized|Intervention Model: Parallel Assignment|Masking: None (Open Label)|Primary Purpose: Treatment
- Enrollment - 65
- Age - 16 Years to 99 Years (Child, Adult, Older Adult)
- Outcome measures - Viral load|Viral load change|Time to clinical improvement (TTCI)|Percentage of progression to supplemental oxygen requirement by day 7|Time to NEWS2 (National Early Warning Score 2) of 3 or more maintained for 24 hours by day 7|Time to clinical failure, defined as the time to death, mechanical ventilation, or ICU admission|Rate of switch to Lopinavir/ritonavir or hydroxychloroquine by day 7|adverse effects|Concentration of Lopinavir/ritonavir and hydroxychloroquine
|
|
NCT04403100
|
Hydroxychloroquine and Lopinavir/ Ritonavir to Improve the Health of People With COVID-19: "The Hope Coalition - 1" |
Recruiting |
Phase 3 |
Jun/03/2020 |
Feb/01/2021 |
- Alternative id - COVID19_AMB_Brasil
- Interventions - Drug: Hydroxychloroquine Sulfate Tablets|Drug: Lopinavir/ Ritonavir Oral Tablet|Drug: Hydroxychloroquine Sulfate Tablets plus Lopinavir/ Ritonavir Oral Tablets|Drug: Placebo
- Study type - Interventional
- Study results - No Results Available
- Locations - CARDRESEARCH - Cardiologia Assistencial e de Pesquisa, Belo Horizonte, Minas Gerais, Brazil|Pontificia Universidade Catolica de Minas Gerais, Belo Horizonte, Minas Gerais, Brazil|Fundo Municipal de Saúde de Betim, Betim, Minas Gerais, Brazil|Universidade Federal de Ouro Preto, Ouro Preto, Minas Gerais, Brazil
- Study designs - Allocation: Randomized|Intervention Model: Factorial Assignment|Masking: Quadruple (Participant, Care Provider, Investigator, Outcomes Assessor)|Primary Purpose: Treatment
- Enrollment - 1968
- Age - 18 Years and older (Adult, Older Adult)
- Outcome measures - Proportion of participants who were hospitalized for progression of COVID-19 disease|Proportion of participants who died due to COVID-19 progression and/ or complications|Proportion of participants with viral load change on 03, 07, 10 and 14 after randomization|Time to clinical improvement|Time to clinical failure|Hospitalization for any cause|Proportion of participants who died due to pulmonary complications|Proportion of participants who died due to cardiovascular complications|Proportion of participants who presented with adverse events|Time to improvement on respiratory scale symptoms|proportion of non-adherent participants to any of study drugs
|
|
NCT04392128
|
Study Evaluating the Efficacy of Hydroxychloroquine and Azithromycine in Patients With COVID-19 and Hematological Malignancies (HYACINTHE) |
Withdrawn |
Phase 2 |
Sep/02/2020 |
Sep/02/2020 |
- Alternative id - 2020-005|2020-002002-45
- Interventions - Drug: Hydroxychloroquine Sulfate 200 MG [Plaquenil]|Drug: Azithromycin 250 MG Oral Capsule|Drug: Placebo oral tablet|Drug: Placebo oral capsule
- Study type - Interventional
- Study results - No Results Available
- Locations - Institut de Cancérologie Strasbourg Europe, Strasbourg, France
- Study designs - Allocation: Randomized|Intervention Model: Parallel Assignment|Masking: Quadruple (Participant, Care Provider, Investigator, Outcomes Assessor)|Primary Purpose: Treatment
- Enrollment - 0
- Age - 18 Years and older (Adult, Older Adult)
- Outcome measures - Evaluation of the efficacy of hydroxychloroquine and azithromyncine on the viral load drop at day 5.|Clinical evolution|Proportion of patients progressing to a severe form|Mortality|Evaluation of viral load drop|Tolerance of study treatment|Evaluation of the seroconversion|NK immunological study|Hospitalisation duration|Impact of the study treatment on the treatment of the hematological disease|Monitoring of the QT space|Dosage of residual concentration of azithromycine and hydroxychloroquine.|T immunological study
|
|
NCT04351191
|
PRophylaxis of Exposed COVID-19 Individuals With Mild Symptoms Using choloroquinE Compounds |
Terminated |
Phase 4 |
Apr/15/2020 |
Aug/30/2020 |
- Alternative id - NBC-COVID1902
- Interventions - Drug: Hydroxychloroquine Sulfate Regular dose|Drug: Hydroxychloroquine Sulfate Loading Dose|Drug: Chloroquine|Drug: Placebo
- Study type - Interventional
- Study results - No Results Available
- Locations - Expo Covid Isolation Center / Mayo Hospital Field Hospital, Lahore, Punjab, Pakistan|Mayo Hospital / King Edward Medical University, Lahore, Punjab, Pakistan|Pakistan Kidney and Liver Institute, Lahore, Punjab, Pakistan|Services Hospital, Lahore, Punjab, Pakistan
- Study designs - Allocation: Randomized|Intervention Model: Parallel Assignment|Masking: Quadruple (Participant, Care Provider, Investigator, Outcomes Assessor)|Primary Purpose: Treatment
- Enrollment - 137
- Age - 20 Years to 50 Years (Adult)
- Outcome measures - RT-PCR result|Progression of symptoms|Mortality
|
|
NCT04316377
|
Norwegian Coronavirus Disease 2019 Study |
Active, not recruiting |
Phase 4 |
Mar/25/2020 |
Mar/03/2025 |
- Alternative id - REC 121446
- Interventions - Drug: Hydroxychloroquine Sulfate
- Study type - Interventional
- Study results - No Results Available
- Locations - Akershus University Hospital, Lørenskog, Norway
- Study designs - Allocation: Randomized|Intervention Model: Parallel Assignment|Masking: None (Open Label)|Primary Purpose: Treatment
- Enrollment - 53
- Age - 18 Years and older (Adult, Older Adult)
- Outcome measures - Rate of decline in SARS-CoV-2 viral load|Change in National Early Warning Score score|Admission to intensive care unit|In-hospital mortality|Duration of hospital admission|Mortality at 30 and 90 days|Clinical status|Change in C-reactive protein concentrations|Change in alanine aminotransferase concentrations|Change in aspartate aminotransferase concentrations|Change in bilirubin concentrations|Change in estimated glomerular filtration rate|Change in cardiac troponin concentrations|Change in natriuretic peptide concentrations
|
|
NCT04328961
|
Hydroxychloroquine for COVID-19 Post-exposure Prophylaxis (PEP) |
Completed |
Phase 2|Phase 3 |
Mar/31/2020 |
Oct/08/2020 |
- Alternative id - STUDY00009750|INV-016204
- Interventions - Drug: Hydroxychloroquine Sulfate|Drug: Ascorbic Acid
- Study type - Interventional
- Study results - Has Results
- Locations - University of California Los Angeles, Los Angeles, California, United States|Tulane, New Orleans, Louisiana, United States|University of Maryland, Baltimore, Baltimore, Maryland, United States|Boston University, Boston, Massachusetts, United States|NYU Langone Health, New York, New York, United States|SUNY Upstate Medical University, Syracuse, New York, United States|University of Washington, Coordinating Center, Seattle, Washington, United States|UW Virology Research Clinic, Seattle, Washington, United States
- Study designs - Allocation: Randomized|Intervention Model: Parallel Assignment|Masking: Double (Participant, Investigator)|Primary Purpose: Prevention
- Enrollment - 943
- Age - 18 Years to 80 Years (Adult, Older Adult)
- Outcome measures - Number of Participants Who Had Polymerase Chain Reaction (PCR) Confirmed SARS-CoV-2 Infection|The Number of Participants Who Had Polymerase Chain Reaction (PCR) Confirmed SARS-CoV-2 Infection|Rate of Participant-reported Adverse Events|Number of Participants Who Had COVID-19 Disease
|
|
NCT04461353
|
A Study to Evaluate the Safety, Tolerability and Pharmacokinetics of Orally Inhaled Aerosolized Hydroxychloroquine Sulfate in Healthy Adult Volunteers |
Completed |
Phase 1 |
Jun/25/2020 |
Aug/17/2020 |
- Alternative id - PUL-01
- Interventions - Drug: Aerolized Hydroxychloroquine Sulfate|Other: Placebo
- Study type - Interventional
- Study results - No Results Available
- Locations - The Rockefeller University, New York, New York, United States
- Study designs - Allocation: Randomized|Intervention Model: Parallel Assignment|Masking: Quadruple (Participant, Care Provider, Investigator, Outcomes Assessor)|Primary Purpose: Treatment
- Enrollment - 12
- Age - 18 Years and older (Adult, Older Adult)
- Outcome measures - Incidences of treatment-emergent adverse events (TEAEs) as assessed by TGSHAAV (September 2007) or CTCAE version 5.0|Change from baseline in clinical laboratory test results for CBC with differential|Incidence of abnormal laboratory test results for CBC with differential at Screening|Incidence of abnormal laboratory test results for CBC with differential - Day 8|Changes from baseline for blood glucose|Incidence of abnormal laboratory test results for chemistry -Screening|Incidence of abnormal laboratory tests results for chemistry - Day 8|Incidence of abnormal laboratory tests results for urinalysis - Screening|Incidence of abnormal laboratory tests results for urinalysis- Day 8|Changes in vital signs from baseline (pre-dose) - respiratory rate|Changes in vital signs from baseline (pre-dose)- temperature|Changes in vital signs from baseline (pre-dose) - seated blood pressure|Changes in vital signs from baseline (pre-dose) - pulse|Changes in vital signs from baseline (pre-dose) - O2 saturation|Incidence of abnormal and physical examinations findings during Screening- general appearance|Incidence of abnormal and physical examinations findings on Day 1 - general appearance|Incidence of abnormal and physical examinations findings on Day 2- general appearance|Incidence of abnormal and physical examinations findings on Day 8- general appearance|Incidence of abnormal and physical examinations findings during Screening- neurological|Incidence of abnormal and physical examinations findings on Day 1- neurological|Incidence of abnormal and physical examinations findings on Day 2- neurological|Incidence of abnormal and physical examinations findings on Day 8- neurological|Incidence of abnormal and physical examinations findings during Screening - heart/cardiovascular|Incidence of abnormal and physical examinations findings on Day 1 - heart/cardiovascular|Incidence of abnormal and physical examinations findings on Day 2 - heart/cardiovascular|Incidence of abnormal and physical examinations findings on Day 8 - heart/cardiovascular|Incidence of abnormal and physical examinations findings during Screening - lungs|Incidence of abnormal and physical examinations findings on Day 1 - lungs|Incidence of abnormal and physical examinations findings on Day 2 - lungs|Incidence of abnormal and physical examinations findings on Day 8 - lungs|Incidence of abnormal and physical examinations findings during Screening- abdomen|Incidence of abnormal and physical examinations findings on Day 1 - abdomen|Incidence of abnormal and physical examinations findings on Day 2- abdomen|Incidence of abnormal and physical examinations findings on Day 8- abdomen|Incidence of abnormal and physical examinations findings during screening- endocrine|Incidence of abnormal and physical examinations findings on Day 1 - endocrine|Incidence of abnormal and physical examinations findings on Day 2- endocrine|Incidence of abnormal and physical examinations findings on Day 8- endocrine|Incidence of abnormal and physical examinations findings during Screening- extremities|Incidence of abnormal and physical examinations findings on Day 1- extremities|Incidence of abnormal and physical examinations findings on Day 2- extremities|Incidence of abnormal and physical examinations findings on Day 8- extremities|Incidence of abnormal and physical examinations findings during Screening- lymphatic|Incidence of abnormal and physical examinations findings on Day 1- lymphatic|Incidence of abnormal and physical examinations findings on Day 2 - lymphatic|Incidence of abnormal and physical examinations findings on Day 8- lymphatic|Incidence of abnormal and physical examinations findings during screening - skin|Incidence of abnormal and physical examinations findings on Day 1 - skin|Incidence of abnormal and physical examinations findings on Day 2 - skin|Incidence of abnormal and physical examinations findings on Day 8 - skin|Changes from baseline for pulmonary function tests (PFTs) - FEV1|Changes from baseline for pulmonary function tests (PFTs) - FVC|Changes from baseline for pulmonary function tests (PFTs) - FEV1/FVC|Changes from baseline for ECG readings - QT interval|Changes from baseline for ECG readings - QTcB Interval|Changes from baseline for ECG readings - QRS duration|Changes from baseline for ECG readings - PR interval|Changes from baseline for ECG readings - heart rate|Incidence of abnormal ECG - Screening|Incidence of abnormal ECG- Day 1|Incidence of abnormal ECG - Day 2|Incidence of abnormal ECG - Day 8|HCQ concentration in whole blood versus time profiles
|