Galidesivir
An adenosine analogue.
General information
Galidesivir on PubChem
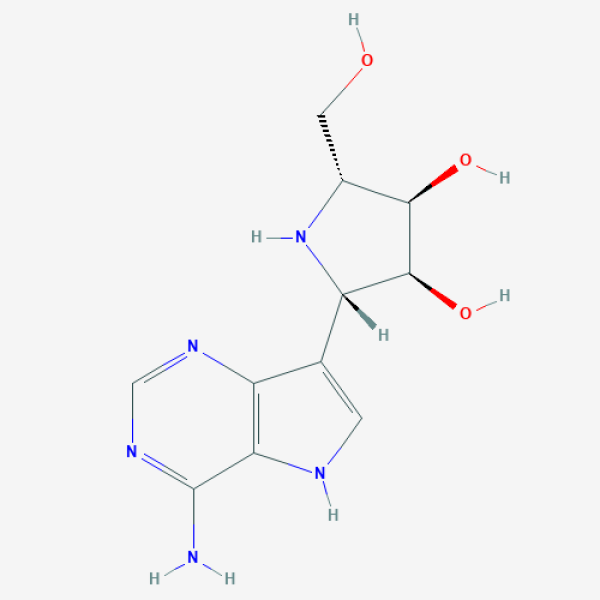
C1=C(C2=C(N1)C(=NC=N2)N)[C@H]3[C@@H]([C@@H]([C@H](N3)CO)O)O
Supporting references
| Link | Tested on | Impact factor | Notes | Publication date |
|---|---|---|---|---|
|
In silico evaluation of potential inhibitory activity of remdesivir, favipiravir, ribavirin and galidesivir active forms on SARS-CoV-2 RNA polymerase
RdRpol Small molecule In silico |
in silico | 2.01 | The triphosphate active metabolite was predicted to inhibit SARS-CoV-2 RNA-dependent RNA polymerase. |
Mar/25/2021 |
AI-suggested references
Clinical trials
| ID | Title | Status | Phase | Start date | Completion date |
|---|---|---|---|---|---|
| NCT03891420 | A Study to Evaluate the Safety, Pharmacokinetics and Antiviral Effects of Galidesivir in Yellow Fever or COVID-19 | Terminated | Phase 1 | Apr/09/2020 | Apr/30/2021 |
|
|||||
