Furosemide
A loop diuretic.
General information
Furosemide is a loop diuretic used for treatment of oedema resulting from a variety of conditions including congestive heart failure, renal failure, liver failure, or high pressure (DrugBank).
Furosemide on PubChem
Furosemide on Wikipedia
Marketed as
DIURAPID; DIURIN; DIURMESSEL; EUTENSIN; FRUMEX; FRUSENEX; FRUSOL; FURO-PUREN; FUROSEMIDE; LASIX; SEGURIL
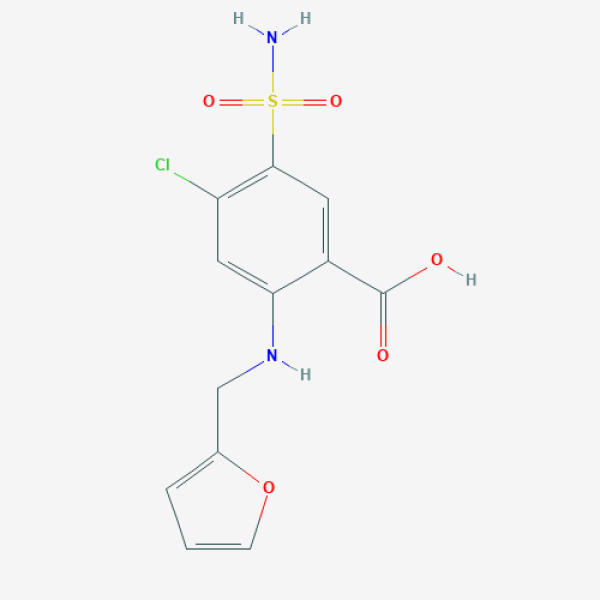
C1=COC(=C1)CNC2=CC(=C(C=C2C(=O)O)S(=O)(=O)N)Cl
Supporting references
| Link | Tested on | Impact factor | Notes | Publication date |
|---|---|---|---|---|
|
Early short-course corticosteroids and furosemide combination to treat non-critically ill COVID-19 patients: An observational cohort study
Small molecule Cohort study |
Patients | 4.84 | Used as an adjunctive therapeutical in corticosteroid-treated patients. Significantly decreased primary outcome in the treated patients (efficacy was significant in the patient subgroup with elevated serum brain natriuretic peptide levels). Sample size: 26 + 93 control. Dosage: 80u202fmg/24u202fh for 4 days. Endpoints: Invasive mechanical ventilation requirement or 28-day mortality (primary). |
Sep/01/2020 |
|
Oral corticoid, aspirin, anticoagulant, colchicine, and furosemide to improve the outcome of hospitalized COVID-19 patients - the COCAA-COLA cohort study
Small molecule Cohort study |
Non-critically ill patients | 6.07 | A five-day treatment with a combination of colchicine, furosemide, prednisolone, acetylsalicylic acid, and a direct anti-Xa drug resulted in a significantly better outcome compared to the control. Sample size: 28 + 40 control. Dosage: 80 mg daily for 5 days. Main outcome: Composite of 28-day mortality, high-flow oxygen therapy requirement or mechanical ventilation requirement. |
Feb/09/2021 |
AI-suggested references
Clinical trials
| ID | Title | Status | Phase | Start date | Completion date |
|---|---|---|---|---|---|
| NCT04588792 | Furosemide as Supportive Therapy for COVID-19 Respiratory Failure | Recruiting | Phase 2|Phase 3 | Apr/16/2021 | Mar/01/2022 |
|
|||||
