Folic acid
A glutamic acid derivative.
General information
Folic acid on PubChem
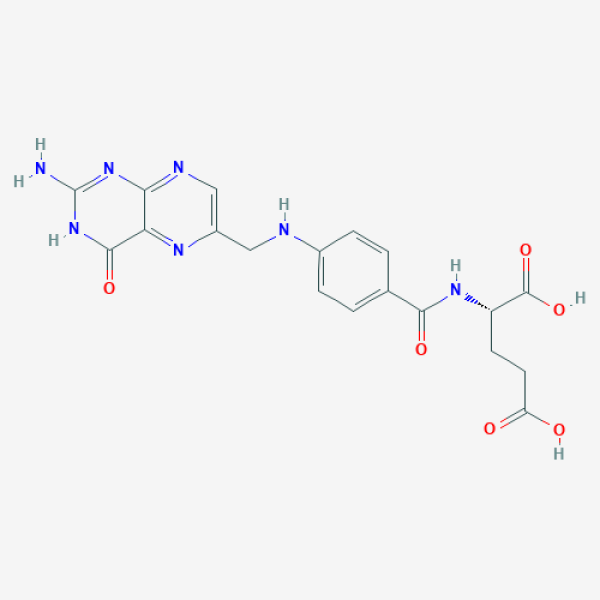
C1=CC(=CC=C1C(=O)N[C@@H](CCC(=O)O)C(=O)O)NCC2=CN=C3C(=N2)C(=O)NC(=N3)N
Supporting references
| Link | Tested on | Impact factor | Notes | Publication date |
|---|---|---|---|---|
|
Evaluation of the potency of FDA-approved drugs on wild type and mutant SARS-CoV-2 helicase (Nsp13)
nsp13 Small molecule In silico |
in silico | 5.16 | Predicted to inhibit the SARS-CoV-2 nsp13 helicase (both wild type and with C17747T and A17858G mutations). |
Sep/24/2020 |
AI-suggested references
Clinical trials
| ID | Title | Status | Phase | Start date | Completion date |
|---|---|---|---|---|---|
| NCT04631536 | Managing Endothelial Dysfunction in COVID-19 : A Randomized Controlled Trial at LAUMC | Active, not recruiting | Phase 3 | Jan/10/2021 | Jul/01/2022 |
|
|||||
| NCT04354428 | Treatment for COVID-19 in High-Risk Adult Outpatients | Active, not recruiting | Phase 2|Phase 3 | Apr/16/2020 | Jan/01/2021 |
|
|||||
