Epoprostenol
A prostacyclin.
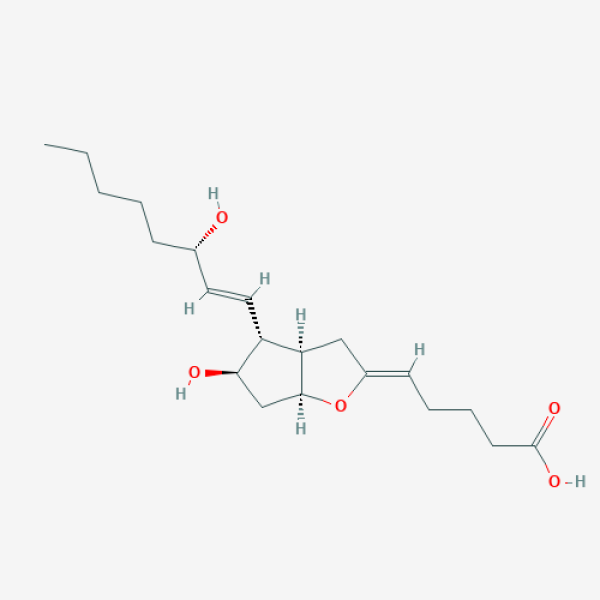
CCCCC[C@@H](/C=C/[C@H]1[C@@H](C[C@H]2[C@@H]1C/C(=C/CCCC(=O)O)/O2)O)O
Supporting references
| Link | Tested on | Impact factor | Notes | Publication date |
|---|---|---|---|---|
|
Superiority of cilostazol among antiplatelet FDA‐approved drugs against COVID 19 Mpro and spike protein: Drug repurposing approach
Spike protein 3CLpro Small molecule In silico Screening |
in silico | 1.90 | Predicted to inhibit the SARS-CoV-2 spike protein. |
Sep/27/2020 |
AI-suggested references
Clinical trials
| ID | Title | Status | Phase | Start date | Completion date |
|---|---|---|---|---|---|
| NCT04452669 | VentaProst in Subjects With COVID-19 Requiring Mechanical Ventilation | Completed | Phase 2 | Sep/15/2020 | Jun/29/2021 |
|
|||||
