Emtricitabine
A nucleoside analogue.
General information
Emtricitabine is a thiacytidine derivate. Its active metabolite inhibits HIV reverse transcriptase (NCIt).
Emtricitabine on PubChem
Emtricitabine on DrugBank
Emtricitabine on Wikipedia
Synonyms
FTC; 2',3'-dideoxy-5-fluoro-3'-thiacytidine
Marketed as
ATRIPLA; BIKTARVY; COMPLERA; COVIRACIL; DESCOVY; EMTRIVA; GENVOYA; ODEFSEY; STRIBILD; TRUVADA
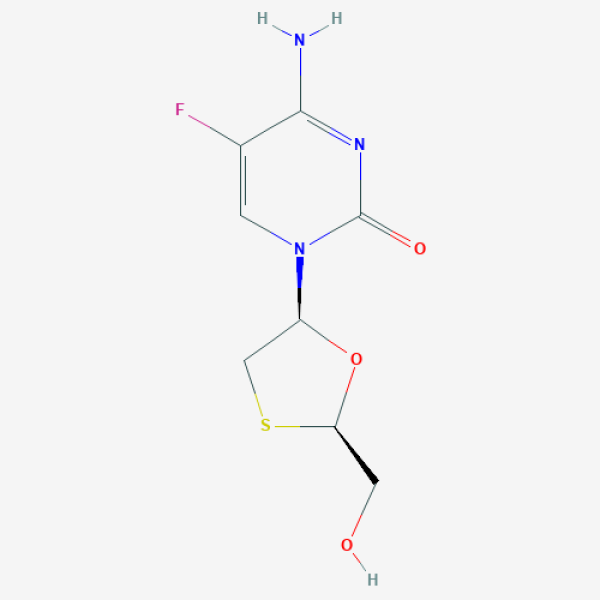
C1[C@H](O[C@H](S1)CO)N2C=C(C(=NC2=O)N)F
Supporting references
| Link | Tested on | Impact factor | Notes | Publication date |
|---|---|---|---|---|
|
Triphosphates of the Two Components in DESCOVY and TRUVADA are Inhibitors of the SARS-CoV-2 Polymerase
Preprint |
in vitro | Apr/05/2020 | ||
|
Effectiveness of rosuvastatin plus colchicine, emtricitabine/tenofovir and combinations thereof in hospitalized patients with COVID-19: a pragmatic, open-label randomized trial
ARDS Small molecule Randomized controlled open trial |
Pneumonia patients | The combined treatment using colchicine, emtricitabine, tenofovir, and rosuvastatin resulted in lower mortality and invasive mechanical ventilation need compared to control. Sample size: (ITT) 159 + 161 control. Dosage: 200 mg daily for 10 days. Main outcome: 28-day mortality. |
Dec/20/2021 |
AI-suggested references
Clinical trials
| ID | Title | Status | Phase | Start date | Completion date |
|---|---|---|---|---|---|
| NCT04890626 | Clinical Trial to Evaluate the Efficacy of Different Treatments in Patients With COVID-19 | Recruiting | Phase 3 | Apr/04/2020 | Nov/30/2022 |
|
|||||
| NCT04334928 | Randomized Clinical Trial for the Prevention of SARS-CoV-2 Infection (COVID-19) in Healthcare Personnel | Completed | Phase 3 | Apr/15/2020 | Jul/11/2021 |
|
|||||
| NCT04712357 | Clinical Experimentation With Tenofovir Disoproxyl Fumarate and Emtricitabine for COVID-19 | Recruiting | Not Applicable | Nov/09/2020 | Jan/01/2023 |
|
|||||
| NCT04359095 | Effectiveness and Safety of Medical Treatment for SARS-CoV-2 (COVID-19) in Colombia | Completed | Phase 2|Phase 3 | Aug/18/2020 | Jun/30/2021 |
|
|||||
| NCT04685512 | Effect of Tenofovir/Emtricitabine in Patients Recently Infected With SARS-COV2 (Covid-19) Discharged Home | Completed | Phase 2|Phase 3 | Nov/18/2020 | May/01/2021 |
|
|||||
| NCT04519125 | Daily Regimen of Tenofovir/Emtricitabine as Prevention for COVID-19 in Health Care Personnel in Colombia | Not yet recruiting | Phase 2|Phase 3 | Aug/30/2020 | Apr/01/2021 |
|
|||||
| NCT04405271 | TAF/FTC for Pre-exposure Prophylaxis of COVID-19 in Healthcare Workers (CoviPrep Study) | Not yet recruiting | Phase 3 | Jul/31/2020 | Nov/15/2020 |
|
|||||
