Ebselen
An organoselenium anti-oxidant.
General information
Ebselen is an organoselenium glutathione peroxidase mimetic with anti-inflammatory, antioxidant, and cytoprotective properties. It also inhibits the activity of multiple enzymes (NCIt).
Ebselen on DrugBank
Ebselen on PubChem
Ebselen on Wikipedia
Synonyms
2-phenyl-1,2-benzoselenazol-3-one; Ebselenum; PZ 51; DR3305; SPI-1005
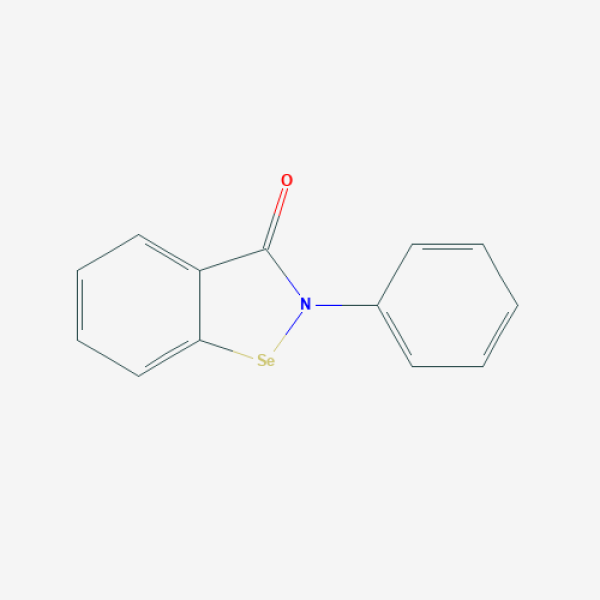
C1=CC=C(C=C1)N2C(=O)C3=CC=CC=C3[Se]2
Supporting references
| Link | Tested on | Impact factor | Notes | Publication date |
|---|---|---|---|---|
|
Discovering drugs to treat coronavirus disease 2019 (COVID-19).
|
in silico | Feb/22/2020 | ||
|
Molecular characterization of ebselen binding activity to SARS-CoV-2 main protease
3CLpro Small molecule In silico |
in silico | 13.12 | Predicted to allosterically block the catalytic site of the SARS-CoV-2 3C-like protease. |
Oct/04/2020 |
|
Structure of Mpro from SARS-CoV-2 and discovery of its inhibitors
3CLpro Crystallization Enzyme assay In vitro In silico |
in silico; in vitro enzyme assay; crystallization; Vero E6 cells | 42.78 | The compound inhibited the SARS-CoV-2 3C-like protease in vitro with IC50 of ca. 0.67 μM and SARS-CoV-2 infection in Vero E6 cells with EC50 of ca. 4.67 μM. |
Jun/11/2020 |
AI-suggested references
Clinical trials
| ID | Title | Status | Phase | Start date | Completion date |
|---|---|---|---|---|---|
| NCT04484025 | SPI-1005 Treatment in Moderate COVID-19 Patients | Enrolling by invitation | Phase 2 | Oct/12/2021 | Aug/01/2022 |
|
|||||
| NCT04483973 | SPI-1005 Treatment in Severe COVID-19 Patients | Enrolling by invitation | Phase 2 | Aug/27/2021 | Aug/01/2022 |
|
|||||
