Ebastine
An H1 antihistamine.
General information
Ebastine is a second-generation antihistamine acing on H1-receptors. It displays efficacy against urticaria and allergic rhinitis with lower sedative effect (Tagawa et al., 2001).
Ebastine on DrugBank
Ebastine on PubChem
Ebastine on Wikipedia
Marketed as
ATMOS; EBAST; EBASTEL FLAS; EBASTEN; EBATIN; EBATROL; EBET; ESTIVANLYO; EVASTEL Z;TEBAST; KESTINE; KESTINELIO; KESTINLYO
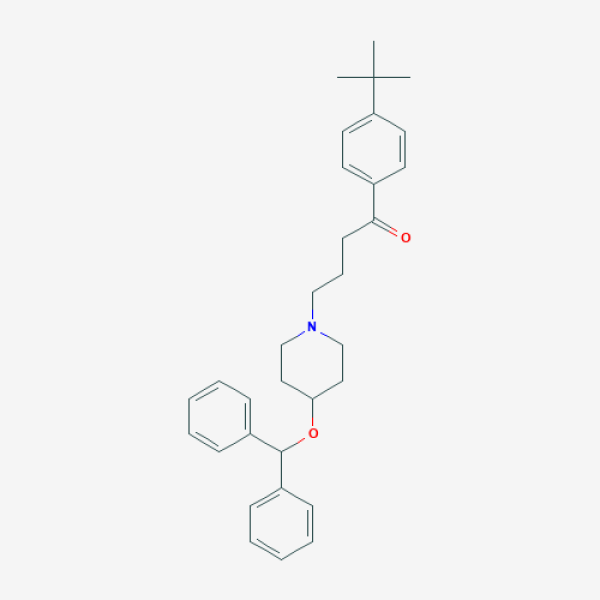
CC(C)(C)C1=CC=C(C=C1)C(=O)CCCN2CCC(CC2)OC(C3=CC=CC=C3)C4=CC=CC=C4
Supporting references
| Link | Tested on | Impact factor | Notes | Publication date |
|---|---|---|---|---|
|
Bepridil is potent against SARS-CoV-2 in vitro
3CLpro Small molecule Enzyme assay In vitro In silico |
in silico; in vitro enzyme assay; Vero E6 cells; A459/ACE2 cells; SARS-CoV-2 live virus | 9.41 | Feb/17/2021 | |
|
Drug repurposing screens reveal cell-type-specific entry pathways and FDA-approved drugs active against SARS-Cov-2
Small molecule In vitro Screening |
Huh7.5 cells; Calu-3 cells; primary normal human bronchial epithelial cells; iPSC-derived AT2 cells; SARS-CoV-2 strain USA WA1/2020 | 8.11 | Inhibited SARS-CoV-2 replication in Huh7.5 cells and also in Calu-3 cells with an SI of >3. |
Mar/23/2021 |
AI-suggested references
Clinical trials
| ID | Title | Status | Phase | Start date | Completion date |
|---|---|---|---|---|---|
| NCT04991246 | Project FLUx COntact-CoVID-19 Faculty of Medicine Paris-Saclay | Not yet recruiting | Not Applicable | Sep/15/2021 | Nov/15/2021 |
|
|||||
