Dutasteride
A selective steroid 5alpha-reductase inhibitor.
General information
Dutasteride is a synthetic compound used for treatment of symptomatic benign prostatic hyperplasia. It blocks conversion of testosterone to 5alpha-dihydrotestosterone (ChEBI).
Dutasteride on DrugBank
Dutasteride on PubChem
Dutasteride on Wikipedia
Marketed as
AVODART; DUTASTERIDE
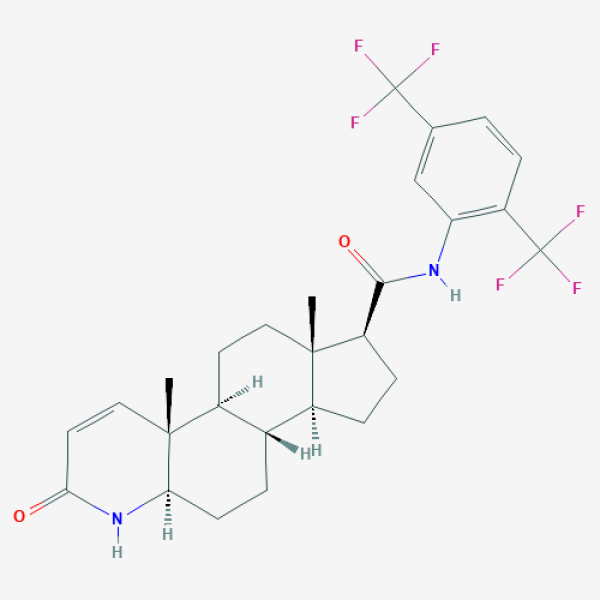
C[C@]12CC[C@H]3[C@H]([C@@H]1CC[C@@H]2C(=O)NC4=C(C=CC(=C4)C(F)(F)F)C(F)(F)F)CC[C@@H]5[C@@]3(C=CC(=O)N5)C
Supporting references
| Link | Tested on | Impact factor | Notes | Publication date |
|---|---|---|---|---|
|
In silico identification of widely used and well-tolerated drugs as potential SARS-CoV-2 3C-like protease and viral RNA-dependent RNA polymerase inhibitors for direct use in clinical trials
|
in silico | 3.31 | Aug/05/2020 | |
|
Early Antiandrogen Therapy With Dutasteride Reduces Viral Shedding, Inflammatory Responses, and Time-to-Remission in Males With COVID-19: A Randomized, Double-Blind, Placebo-Controlled Interventional Trial (EAT-DUTA AndroCoV Trial – Biochemical)
Small molecule Randomized controlled double-blind trial Moderate severity Mild severity |
Male outpatients | Outpatients in the treatment group had statistically significantly shortened COVID-19 duration, reduced viral shedding, improved oxygen saturation, and decreased inflammatory marker levels. Sample size: 43 + 44 placebo (both groups undergoing early nitazoxanide and azithromycin therapy). Dosage: 0.5 mg daily or until full COVID-19 remission. |
Feb/01/2021 |
AI-suggested references
Clinical trials
| ID | Title | Status | Phase | Start date | Completion date |
|---|---|---|---|---|---|
| NCT04729491 | EAT-DUTA AndroCoV Trial | Completed | Phase 2|Phase 3 | Jun/30/2020 | Oct/07/2020 |
|
|||||
