Danoprevir
A hepatitis C virus protease inhibitor.
General information
Danoprevir is an experimental anti-hepatitis C virus drug (DrugBank).
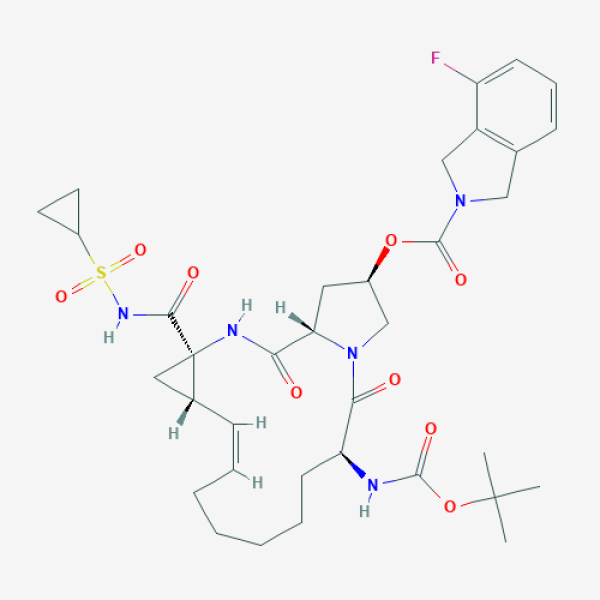
CC(C)(C)OC(=O)N[C@H]1CCCCC/C=C\[C@@H]2C[C@]2(NC(=O)[C@@H]3C[C@H](CN3C1=O)OC(=O)N4CC5=C(C4)C(=CC=C5)F)C(=O)NS(=O)(=O)C6CC6
Supporting references
| Link | Tested on | Impact factor | Notes | Publication date |
|---|---|---|---|---|
|
Predicting commercially available antiviral drugs that may act on the novel coronavirus (2019-nCoV), Wuhan, China through a drug-target interaction deep learning model
Preprint In silico |
in silico | Feb/02/2020 | ||
|
First Clinical Study Using HCV Protease Inhibitor Danoprevir to Treat Naive and Experienced COVID-19 Patients
Preprint |
Patients | in combination with ritonavir |
Mar/24/2020 | |
|
Molecular modeling evaluation of the binding effect of five protease inhibitors to COVID-19 main protease
3CLpro Small molecule In silico |
in silico | 1.77 | Predicted to inhibit the SARS-CoV-2 3C-like protease. |
Dec/11/2020 |
AI-suggested references
Clinical trials
| ID | Title | Status | Phase | Start date | Completion date |
|---|---|---|---|---|---|
| NCT04291729 | Evaluation of Ganovo (Danoprevir ) Combined With Ritonavir in the Treatment of SARS-CoV-2 Infection | Completed | Phase 4 | Feb/17/2020 | Mar/19/2020 |
|
|||||
| NCT04345276 | Efficacy and Safety of Ganovo (Danoprevir) Combined With Ritonavir in the Treatment of SARS-CoV-2 Infection | Completed | Phase 4 | Mar/18/2020 | Apr/15/2020 |
|
|||||
