Crocetin
A natural vitamin A analogue.
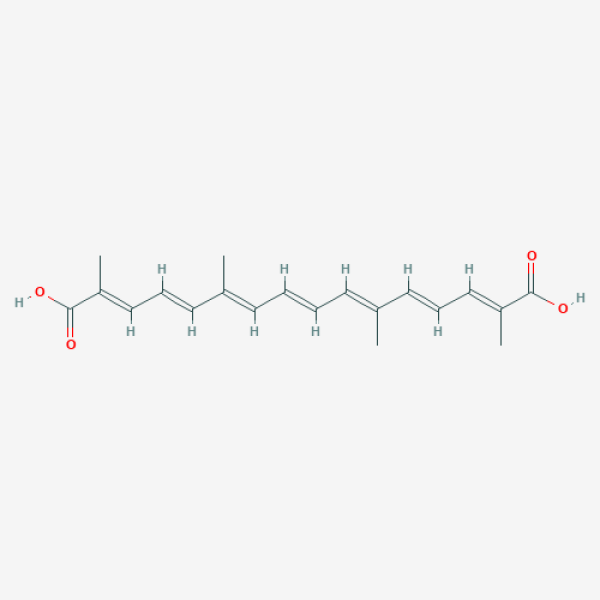
C/C(=C\C=C\C=C(\C=C\C=C(\C(=O)O)/C)/C)/C=C/C=C(/C(=O)O)\C
Supporting references
| Link | Tested on | Impact factor | Notes | Publication date |
|---|---|---|---|---|
|
Investigation on penetration of saffron components through lipid bilayer bound to spike protein of SARS-CoV-2 using steered molecular dynamics simulation
3CLpro Small molecule In silico |
in silico | Predicted to bind the SARS-CoV-2 3C-like protease and have the capability to cross the lipid bilayer. |
Dec/12/2020 |
AI-suggested references
| Link | Publication date |
|---|---|
|
Liposomal encapsulation of trans-crocetin enhances oxygenation in patients with COVID-19-related ARDS receiving mechanical ventilation
|
Jun/24/2021 |
Clinical trials
| ID | Title | Status | Phase | Start date | Completion date |
|---|---|---|---|---|---|
| NCT04378920 | A Study of Liposomal Trans Crocetin, LEAF-4L6715, in Patients With Acute Respiratory Distress Syndrome Due to COVID-19, Sepsis or Other Causes | Completed | Phase 1|Phase 2 | Apr/14/2020 | Aug/24/2021 |
|
|||||
| NCT04573322 | Safety and Efficacy of Trans Sodium Crocetinate (TSC) in SARS-CoV-2 (COVID-19) Infected Subjects | Completed | Phase 1|Phase 2 | Sep/10/2020 | Apr/29/2021 |
|
|||||
