COVID-19 Vaccines Comparison: AstraZeneca, Moderna, and Pfizer/BioNTech
Author: Ivana Mišová, PhD.
Published at: 01/25/2021
At this time – the beginning of 2021 – many countries have approved more than one COVID-19 vaccine. Finally, having an effective vaccine against COVID-19 is undoubtedly a great success. Nevertheless, countries are selective in which vaccine to order and in what quantities. What are the differences between the available vaccines? And is it possible to tell which one is the best?
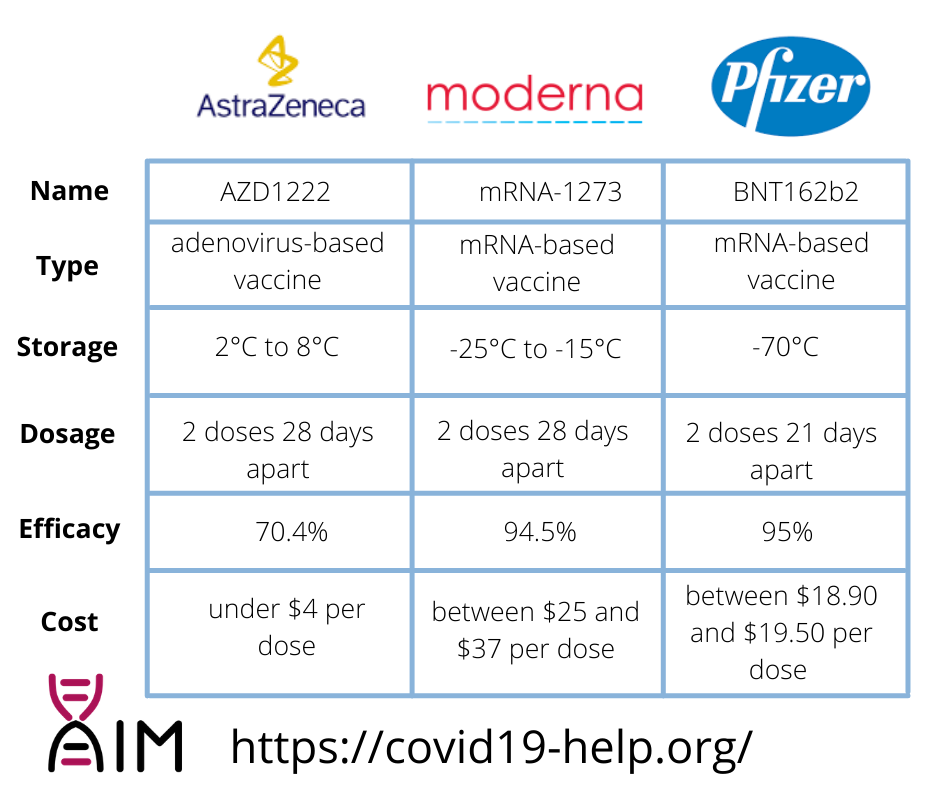
The Pfizer/BioNTech vaccine BNT162b2 was the topic of the previous blog. It was approved on December 2, 2020, in the UK, making it the first “Western” vaccine to receive authorization1. It is now approved in multiple countries, including Canada, the USA, the EU, Chile, Mexico, Ecuador, Panama, and Costa Rica2. It is an mRNA vaccine and must be kept at -70°C and used quickly once thawed and diluted3. Its price is reported to be $19.50 per dose for the 100 million doses ordered by the USA and 15.50 euro ($18.90) per dose for the 300 million doses ordered by the EU4. To obtain the optimal protection the vaccine can offer, each person is injected with two doses 21 days apart5. Data from the Phase 3 clinical study demonstrated an efficacy rate of 95% in participants without prior SARS-CoV-2 infection6. Moreover, preliminary data suggest that the vaccine can neutralize the novel, easily transmissible SARS-CoV-2 strain with the Y501N mutation7.
The ModernaTX, Inc. COVID-19 vaccine is mRNA-1273. It was first approved on December 18, 2020, in the USA8. It is now approved in multiple countries, including Canada, Israel, the EU, the UK, and Switzerland9. It is also an mRNA vaccine; however, unlike the Pfizer mRNA vaccine, it can be stored between -25°C to -15°C and even refrigerated for up to 30 days before the first use10. The Moderna vaccine price is reported to be between $25 and $37 per dose, depending on the amount ordered11. To obtain the vaccine’s optimal protection, each person is injected with two doses one month (28 days) apart12. Data from the Phase 3 clinical study demonstrated an efficacy rate of 94.5%13. Its efficacy against novel variants of the SARS-CoV-2 is currently being tested, but the vaccine is expected to remain equally effective14.
The COVID-19 vaccine developed by AstraZeneca in collaboration with the University of Oxford is called AZD1222. It was first approved on December 30, 2020, in the UK15. It is now approved in multiple countries, including Argentina, Dominican Republic, El Salvador, India, Mexico, and Morocco, but not yet in the USA or the EU16. Unlike the vaccines from Pfizer and Moderna, this is an adenovirus-based vaccine. It uses a replication-deficient chimpanzee viral vector based on a weakened version of a common cold virus (adenovirus) that causes infections in chimpanzees and contains the genetic material of the SARS-CoV-2 virus spike protein. After vaccination, the surface spike protein is produced, priming the immune system to attack the SARS-CoV-2 virus if it later infects the body17. This vaccine can be stored at 2°C to 8°C. AstraZeneca is a member of a global initiative called Covax, aiming to distribute two billion vaccine doses to 92 low- and middle-income countries at no more than $3 a dose18. Its agreed purchase price with the US government is also under $4 per dose. Interim analysis of clinical trials of AZD1222 showed vaccine efficacy of 90% when AZD1222 was given as a half dose followed by a full dose at least one month apart, and 62.1% efficacy when given as two full doses at least one month apart, resulting in an average efficacy of 70.4%19. AstraZeneca says that its vaccine should be effective against the new coronavirus variant and studies are underway to analyze the impact of the mutation thoroughly20.
The key differences between the vaccines that can affect the countries’ choice are the vaccines’ efficacy, cost, and temperature requirements for storage and distribution. The Pfizer vaccine has the highest efficacy of the three but the most demanding temperature requirements. The Moderna vaccine is also highly effective and has less extreme temperature requirements, but it is the most expensive of the three. The AstraZeneca vaccine is the cheapest and easiest to store but has the lowest efficacy of the three compared vaccines. The latest is also yet to be approved in the USA or the EU. Moreover, the effectiveness of all these vaccines against the novel emerging SARS-CoV-2 strains needs to be tested. However, it is essential to note that the vaccines are not really up against each other, but they are all going to play key roles in ending this pandemic.
References
- https://www.gov.uk/government/publications/regulatory-approval-of-pfizer-biontech-vaccine-for-covid-19
- https://www.canada.ca/en/health-canada/services/drugs-health-products/covid19-industry/drugs-vaccines-treatments/vaccines/pfizer-biontech.html, https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/pfizer-biontech-covid-19-vaccine, https://www.ema.europa.eu/en/news/ema-recommends-first-covid-19-vaccine-authorisation-eu, https://www.ema.europa.eu/en/news/ema-recommends-first-covid-19-vaccine-authorisation-eu
- https://www.fda.gov/media/144413/download
- https://www.reuters.com/article/us-health-coronavirus-eu-vaccine-prices/eu-agreed-1550-euros-per-dose-for-pfizer-vaccine-document-idUSKBN28V0Y6
- https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/Pfizer-BioNTech.html
- Polack, F. P., Thomas, S. J., Kitchin, N., Absalon, J., Gurtman, A., Lockhart, S., … Gruber, W. C. (2020). Safety and efficacy of the BNT162b2 mRNA Covid-19 Vaccine. New England Journal of Medicine, 383(27), 2603–2615. https://doi.org/10.1056/NEJMoa2034577
- https://www.biorxiv.org/content/10.1101/2021.01.07.425740v1
- https://www.fda.gov/news-events/press-announcements/fda-takes-additional-action-fight-against-covid-19-issuing-emergency-use-authorization-second-covid
- https://www.canada.ca/en/health-canada/services/drugs-health-products/covid19-industry/drugs-vaccines-treatments/authorization/list-drugs.html, https://investors.modernatx.com/news-releases/news-release-details/israeli-ministry-health-authorizes-covid-19-vaccine-moderna-use, https://investors.modernatx.com/news-releases/news-release-details/european-commission-authorizes-covid-19-vaccine-moderna-europe, https://investors.modernatx.com/news-releases/news-release-details/united-kingdom-medicines-and-healthcare-products-regulatory, https://investors.modernatx.com/news-releases/news-release-details/swissmedic-authorizes-covid-19-vaccine-moderna-use-switzerland
- https://www.fda.gov/media/144637/download
- https://www.reuters.com/article/uk-health-coronavirus-moderna-eu/moderna-to-charge-25-37-for-covid-19-vaccine-ceo-tells-paper-idUKKBN2810WK
- https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/Moderna.html
- https://investors.modernatx.com/news-releases/news-release-details/modernas-covid-19-vaccine-candidate-meets-its-primary-efficacy
- https://investors.modernatx.com/news-releases/news-release-details/statement-variants-sars-cov-2-virus
- https://www.astrazeneca.com/content/astraz/media-centre/press-releases/2020/astrazenecas-covid-19-vaccine-authorised-in-uk.html
- https://www.astrazeneca.com/content/astraz/media-centre/press-releases/2021/serum-institute-of-india-obtains-emergency-use-authorisation-in-india-for-astrazenecas-covid-19-vaccine.html
- https://www.astrazeneca.com/media-centre/press-releases/2020/azd1222hlr.html
- https://observer.com/2020/11/covid19-vaccine-price-pfizer-moderna-astrazeneca-oxford/
- Voysey et al., “Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK,” Lancet, vol. 397, no. 10269, p. 2021, Jan. 2020.
- https://www.reuters.com/article/health-coronavirus-astrazeneca-vaccine/update-1-astrazeneca-says-its-vaccine-should-be-effective-against-new-coronavirus-variant-idUKL1N2J21YQ
