Cobicistat
A cytochrome P450 3A inhibitor.
General information
Cobicistat is a cytochrome P450 3A inhibitor which is used as a pharmacokinetic enhancer for certain HIV-1 antivirals (NCIt).
Cobicistat on DrugBank
Cobicistat on PubChem
Cobicistat on Wikipedia
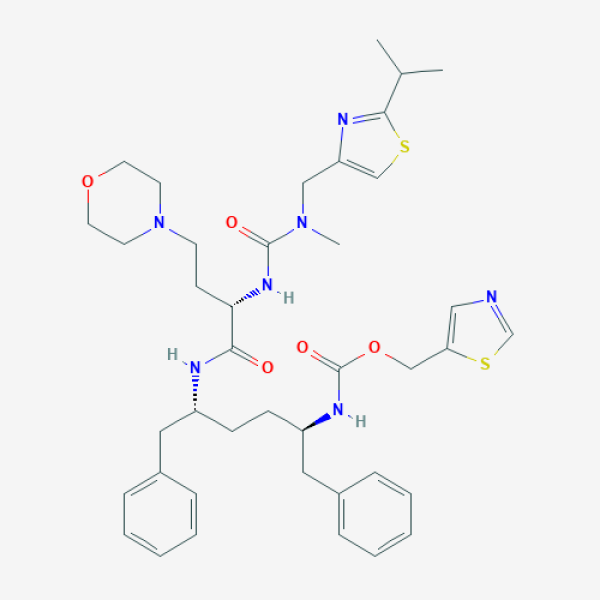
CC(C)C1=NC(=CS1)CN(C)C(=O)N[C@@H](CCN2CCOCC2)C(=O)N[C@H](CC[C@H](CC3=CC=CC=C3)NC(=O)OCC4=CN=CS4)CC5=CC=CC=C5
Supporting references
AI-suggested references
Clinical trials
| ID | Title | Status | Phase | Start date | Completion date |
|---|---|---|---|---|---|
| NCT04425382 | Darunavir/Cobicistat vs. Lopinavir/Ritonavir in COVID-19 Pneumonia in Qatar | Recruiting | Mar/01/2020 | Sep/01/2020 | |
|
|||||
| NCT04252274 | Efficacy and Safety of Darunavir and Cobicistat for Treatment of COVID-19 | Recruiting | Phase 3 | Jan/30/2020 | Dec/31/2020 |
|
|||||
