Chlorpromazine
A postsynaptic dopamine receptor.
General information
Chlorpromazine is a phenothiazine antipsychotic drug. It blocks postsynaptic dopamine receptors in the brain (NCIt).
Chlorpromazine on DrugBank
Chlorpromazine on PubChem
Chlorpromazine on Wikipedia
Synonyms
Chlorpromazine hydrochloride
Marketed as
CHLORPROMAZINE HYDROCHLORIDE
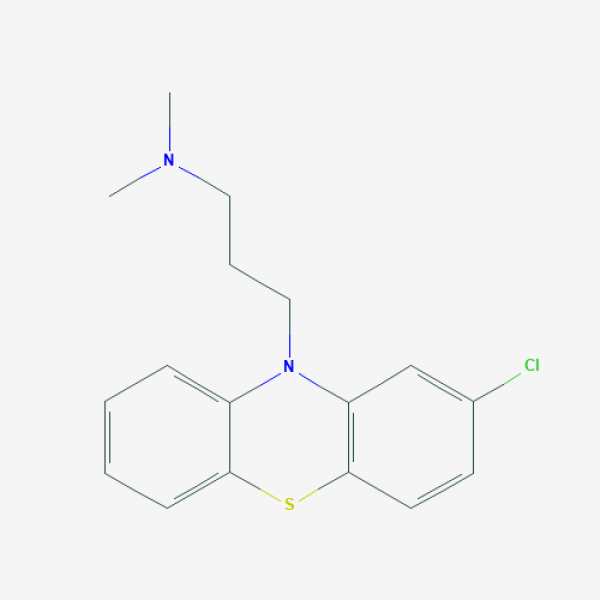
CN(C)CCCN1C2=CC=CC=C2SC3=C1C=C(C=C3)Cl
Supporting references
| Link | Tested on | Impact factor | Notes | Publication date |
|---|---|---|---|---|
|
Inhibition of the replication of SARS-CoV-2 in human cells by the FDA-approved drug chlorpromazine
Preprint |
human A549-ACE2 cells | May/06/2020 | ||
|
Broad anti-coronaviral activity of FDA approved drugs against SARS-CoV-2 in vitro and SARS-CoV in vivo
Small molecule Animal model |
BALB/c mice; A549 lung adenocarcinoma cells expressing ACE2; Vero E6 cells; SARS-CoV-2 strain MA15 | 4.32 | Protected mice infected by mouse-adapded SARS-CoV-2 from clinical disease. Did not inhibit viral replication in the lungs, however. Inhibits the production of infectious viral particles in vitro and decreases viral gene RNA levels. |
Aug/19/2020 |
|
Screened antipsychotic drugs inhibit SARS-CoV-2 binding with ACE2 in vitro
ACE2 Biophysical assay In vitro In silico |
in silico; in vitro biophysical assay; ACE2 high-expressing HEK293T cells; ACE2-HEK293T cell membrane chromatography | 3.65 | Bound to ACE2 and cell membranes of ACE2-expressing cells and reduced SARS-CoV-2 Spike pseudotyped virus entry ratio in vitro. |
Dec/10/2020 |
AI-suggested references
Clinical trials
| ID | Title | Status | Phase | Start date | Completion date |
|---|---|---|---|---|---|
| NCT04354805 | Administration of Chlorpromazine as a Treatment for COVID-19 | Not yet recruiting | Phase 2|Phase 3 | Aug/01/2020 | Nov/01/2020 |
|
|||||
| NCT04366739 | Repurposing of Chlorpromazine in Covid-19 Treatment | Not yet recruiting | Phase 3 | Apr/29/2020 | Sep/30/2020 |
|
|||||
