Cetirizine
A histamin H1-receptor antagonist.
General information
Cetirizine Hydrochloride is a synthetic histamine H1-receptor antagonist. It is used for relief of symptoms of seasonal or perennial allergic rhinitis and of chronic urticaria (NCIt).
Cetirizine on DrugBank
Cetirizine on PubChem
Cetirizine on Wikipedia
Marketed as
ALERLISIN; CETRYN; CETIRIZINE (HYDROCHLORIDE); FORMISTIN; HITRIZIN; QUZYTTIR; VIRLIX; ZIRTEK; ZYRTEK; ZYRLEX
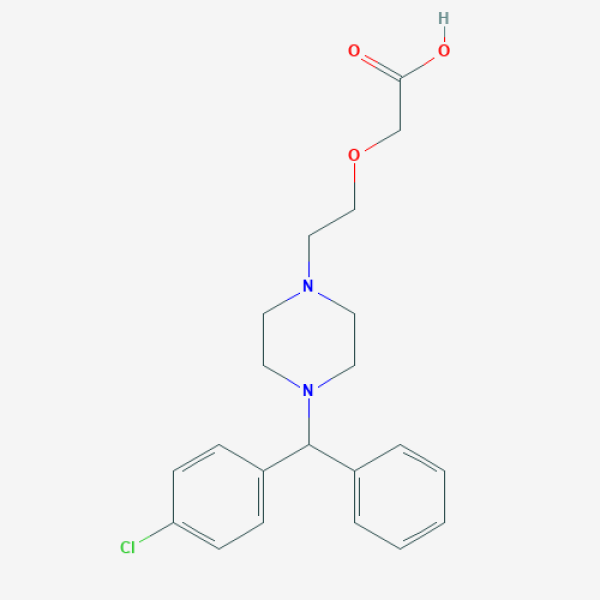
C1CN(CCN1CCOCC(=O)O)C(C2=CC=CC=C2)C3=CC=C(C=C3)Cl
Supporting references
| Link | Tested on | Impact factor | Notes | Publication date |
|---|---|---|---|---|
|
Dual-histamine receptor blockade with cetirizine - famotidine reduces pulmonary symptoms in COVID-19 patients
Severe severity Small molecule Critical severity Cohort study |
Patients | 2.68 | In combination with famotidine reduced progression in symptom severity. Sample size: 110. Dosage: 10 mg twice daily. Endpoints: Increase in the rate of discharge; reduction in ventilation requirements; reduction in inpatient mortality rate; reduction in duration of hospitalization. |
Aug/29/2020 |
AI-suggested references
| Link | Publication date |
|---|---|
|
Antihistamines and azithromycin as a treatment for COVID-19 on primary health care - A retrospective observational study in elderly patients
|
Jan/16/2021 |
Clinical trials
| ID | Title | Status | Phase | Start date | Completion date |
|---|---|---|---|---|---|
| NCT04836806 | Cetirizine and Famotidine for COVID-19 | Withdrawn | Phase 4 | Aug/01/2021 | Jul/01/2022 |
|
|||||
