Canrenone
A mineralcorticoid receptor antagonist.
General information
Canrenone is an aldosterone antagonist. Its activity leads to increase in sodium excretion and inhibition of potassium excretion in the kidneys (NCIt).
Canrenone on DrugBank
Canrenone on PubChem
Canrenone on Wikipedia
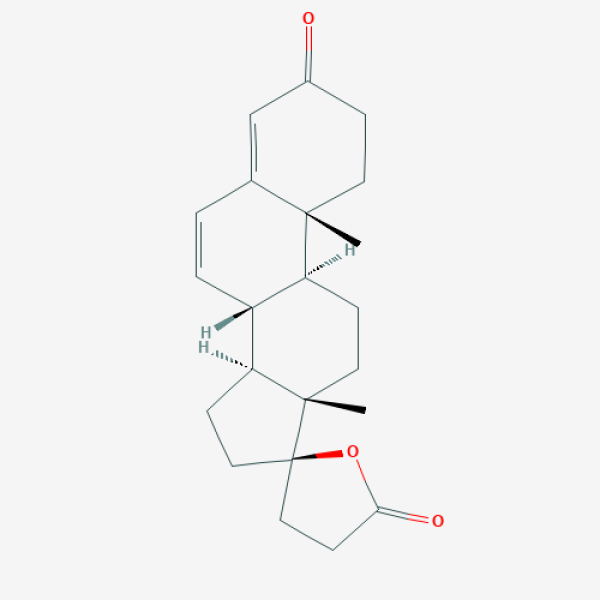
C[C@]12CCC(=O)C=C1C=C[C@@H]3[C@@H]2CC[C@]4([C@H]3CC[C@@]45CCC(=O)O5)C
Supporting references
| Link | Tested on | Impact factor | Notes | Publication date |
|---|---|---|---|---|
|
Hijacking SARS-Cov-2/ACE2 receptor interaction by natural and semi-synthetic steroidal agents acting on functional pockets on receptor binding region
ACE2 Preprint |
in vitro | Jun/11/2020 | ||
|
The Efficacy of the Mineralcorticoid Receptor Antagonist Canrenone in COVID-19 Patients
Severe severity Small molecule Moderate severity Cohort study |
Patients | 3.30 | Significant improvement in clinical status (including oxygenation, inflammation parameters, and blood pressure) and increase in event-free rate and survival rate. Sample size: 30 + 39 (RAAS inhibitors and/or vasodilatators). Dosage: 200 mg IV daily for 14 ± 11 days (at least 2 consecutive). |
Sep/11/2020 |
Clinical trials
| ID | Title | Status | Phase | Start date | Completion date |
|---|---|---|---|---|---|
| NCT04977960 | Efficacy of Canrenone as add-on Treatment in Moderate to Severe ARDS in COVID-19 | Not yet recruiting | Phase 2 | Sep/01/2021 | Dec/01/2022 |
|
|||||
