Cannabidiol
A non-psychoactive phytocannabinoid.
General information
Cannabidiol is a phytocannabinoid without psychoactive activity. It has been shown to possess analgesic, anti-inflammatory, antineoplastic, and chemopreventive properties (NCIt).
Cannabidiol on DrugBank
Cannabidiol on PubChem
Cannabidiol on Wikipedia
Synonyms
CBD
Marketed as
EPIDIOLEX
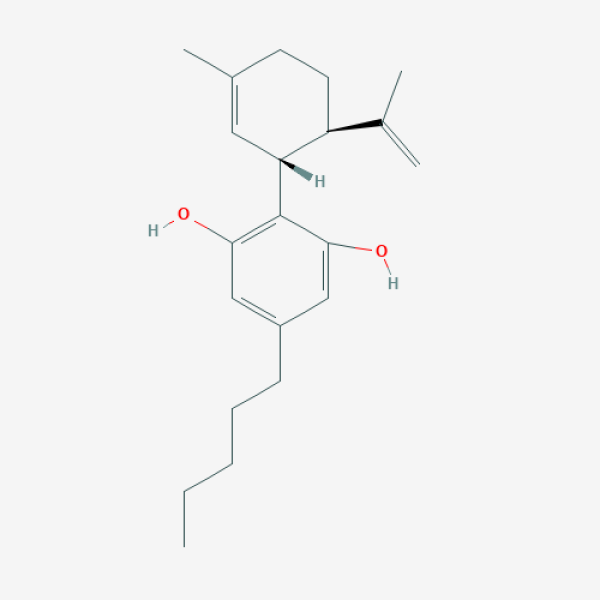
CCCCCC1=CC(=C(C(=C1)O)[C@@H]2C=C(CC[C@H]2C(=C)C)C)O
Supporting references
| Link | Tested on | Impact factor | Notes | Publication date |
|---|---|---|---|---|
|
Assessment of antiviral potencies of cannabinoids against SARS-CoV-2 using computational and in vitro approaches
3CLpro Small molecule In vitro In silico |
in silico; Vero cells | 5.16 | Inhibited SARS-CoV-2 in vitro (IC50u202f=u202f7.91u202fμM in Vero cells). |
Dec/05/2020 |
|
Cannabis compounds exhibit anti-inflammatory activity in vitro in COVID-19-related inflammation in lung epithelial cells and pro-inflammatory activity in macrophages
Small molecule In vitro |
A549 cells; KG1 cells | 4.00 | The compound reduced IL-8 levels in A549 cells when used in concentration of 3 μg/mL. Lower or higher concentrations did not result in IL-8 levels' decrease, however (bell-shaped dose-response). Combination of CBD with other bioactive constituents of Cannabis extracts might lead to increase of pro-inflammatory cytokine production in macrophages. |
Jan/14/2021 |
AI-suggested references
Clinical trials
| ID | Title | Status | Phase | Start date | Completion date |
|---|---|---|---|---|---|
| NCT04603781 | CBD Oil for Reducing Emotional Impact of COVID-19 | Recruiting | Phase 2|Phase 3 | Dec/04/2020 | Dec/31/2021 |
|
|||||
| NCT04504877 | Burnout and Distress preventiOn With caNnabidiol in Front-line Health Care workerS deAling wIth COVID-19 | Completed | Phase 2|Phase 3 | Jun/16/2020 | Dec/16/2020 |
|
|||||
| NCT04615949 | Cannabidiol in Patients With COVID-19 and Cardiovascular Disease or Risk Factors | Recruiting | Phase 2|Phase 3 | Apr/30/2021 | Apr/01/2022 |
|
|||||
| NCT04731116 | Cannabidiol Treatment for Severe and Critical Coronavirus (COVID-19) Pulmonary Infection | Recruiting | Phase 1|Phase 2 | Jan/10/2021 | Apr/30/2022 |
|
|||||
| NCT04777981 | (CBDRA60) to Prevent or Reduce Symptoms of COVID-19 and Prevention of Post-Acute Sequelae of SARS-CoV-2 Infection PASC | Not yet recruiting | Not Applicable | Jul/01/2022 | Dec/28/2022 |
|
|||||
| NCT04467918 | CANnabiDiol for CoviD-19 pATiEnts With Mild to Moderate Symptoms | Active, not recruiting | Phase 2|Phase 3 | Jul/06/2020 | Nov/16/2021 |
|
|||||
| NCT04686539 | Synthetic CBD as a Therapy for COVID-19 | Recruiting | Phase 1 | Jan/20/2021 | Jan/01/2022 |
|
|||||
| NCT04997395 | Feasibility of Cannabidiol for the Treatment of Long COVID | Not yet recruiting | Phase 2 | Jan/01/2022 | Dec/01/2022 |
|
|||||
