Bromhexine hydrochloride
A mucolytic agent and a TMPRSS2 inhibitor.
General information
Bromhexine hydrochloride is a secretolytic/mucolytic agent (NCIt) and a TMPRSS2 inhibitor (Lucas et al., 2014).
Bromhexine (hydrochloride) on DrugBank
Bromhexine hydrochloride on PubChem
Bromhexine (hydrochloride) on Wikipedia
Marketed as
AMIOREL; BISOLMED; BISOLVON; FLUIBRON
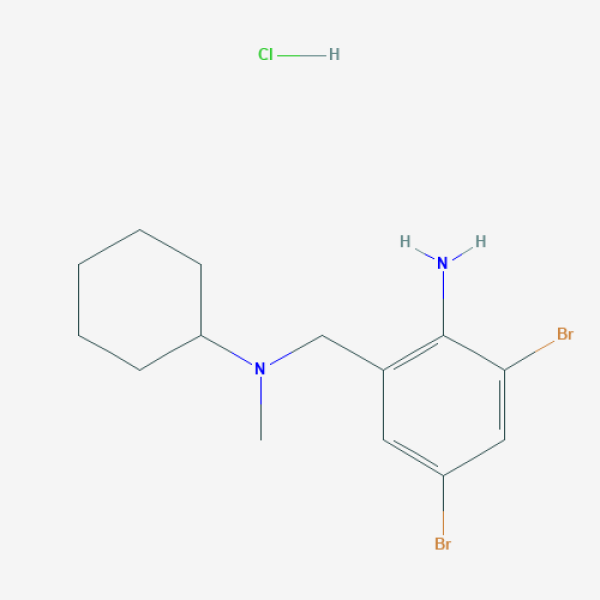
CN(CC1=C(C(=CC(=C1)Br)Br)N)C2CCCCC2.Cl
Supporting references
| Link | Tested on | Impact factor | Notes | Publication date |
|---|---|---|---|---|
|
Alpha 1 Antitrypsin is an Inhibitor of the SARS-CoV2-Priming Protease TMPRSS2
TMPRSS2 Preprint |
HEK-293T cell culture | inhibited TMPRSS2 proteolytic function |
Oct/07/2020 | |
|
Bromhexine Hydrochloride Tablets for the Treatment of Moderate COVID‐19: An Open‐label Randomized Controlled Pilot Study
TMPRSS2 Small molecule Randomized controlled open trial Moderate severity Mild severity |
Patients | 3.37 | Improvement (statistically not significant) in chest computer tomography findings, oxygen therapy need and the discharge rate within 20 days. Sample size: 12 + 6 control. Dosage: 32 mg twice daily for 14 days. |
Sep/03/2020 |
|
Effect of bromhexine on clinical outcomes and mortality in COVID-19 patients: A randomized clinical trial
Small molecule Randomized controlled open trial |
Patients | 3.19 | A significantly lower rate of ICU admission, intubation, and 28-day mortality in the bromhexine treatment group compared to the control group. Sample size: 39 + 39 control. Dosage: 8 mg three times a day for 2 weeks. |
Jul/19/2020 |
AI-suggested references
Clinical trials
| ID | Title | Status | Phase | Start date | Completion date |
|---|---|---|---|---|---|
| NCT04405999 | Prevention of Infection and Incidence of COVID-19 in Medical Personnel Assisting Patients With New Coronavirus Disease | Completed | Phase 4 | May/14/2020 | Aug/31/2020 |
|
|||||
| NCT04273763 | Evaluating the Efficacy and Safety of Bromhexine Hydrochloride Tablets Combined With Standard Treatment/ Standard Treatment in Patients With Suspected and Mild Novel Coronavirus Pneumonia (COVID-19) | Active, not recruiting | Not Applicable | Feb/16/2020 | Jun/01/2020 |
|
|||||
