Bicalutamide
A non-steroid anti-androgen drug.
General information
Bicalutamide is a synthetic non-steroid cytosolic androgen receptor competitive inhibitor (NCIt). It is used in the prostate cancer treatment (DrugBank).
Bicalutamide on PubChem
Bicalutamide on Wikipedia
Marketed as
BICALUTAMIDE; CASODEX
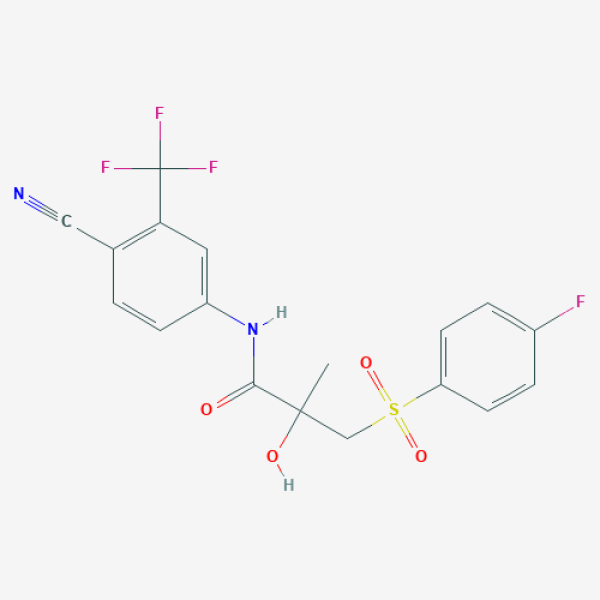
CC(CS(=O)(=O)C1=CC=C(C=C1)F)(C(=O)NC2=CC(=C(C=C2)C#N)C(F)(F)F)O
Supporting references
| Link | Tested on | Impact factor | Notes | Publication date |
|---|---|---|---|---|
|
Conserved interactions required for inhibition of the main protease of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)
3CLpro Small molecule Enzyme assay In vitro In silico |
in silico; in vitro enzyme assay | 4.00 | Displayed inhibitory activity against SARS-CoV-2 3C-like protease in vitro (over 30% at 50 μM). More accurate measurements are needed, however. |
Nov/30/2020 |
AI-suggested references
Clinical trials
| ID | Title | Status | Phase | Start date | Completion date |
|---|---|---|---|---|---|
| NCT04509999 | Bicalutamide to Block TMPRSS2 in Males With COVID-19 Infection | Withdrawn | Phase 3 | Oct/26/2020 | Jun/30/2021 |
|
|||||
| NCT04652765 | Camostat With Bicalutamide for COVID-19 | Terminated | Phase 1 | Feb/03/2021 | Sep/15/2021 |
|
|||||
| NCT04374279 | Trial to Promote Recovery From COVID-19 With Endocrine Therapy | Withdrawn | Phase 2 | Apr/01/2021 | Jan/01/2022 |
|
|||||
