Bemcentinib
A tyrosine kinase inhibitor.
General information
Bemcentinib is an AXL receptor tyrosine kinase selective inhibitor. It has been investigated for its antineoplastic activity (NCIt).
Bemcentinib on DrugBank
Bemcentinib on PubChem
Bemcentinib on Wikipedia
Synonyms
R428; BGB324
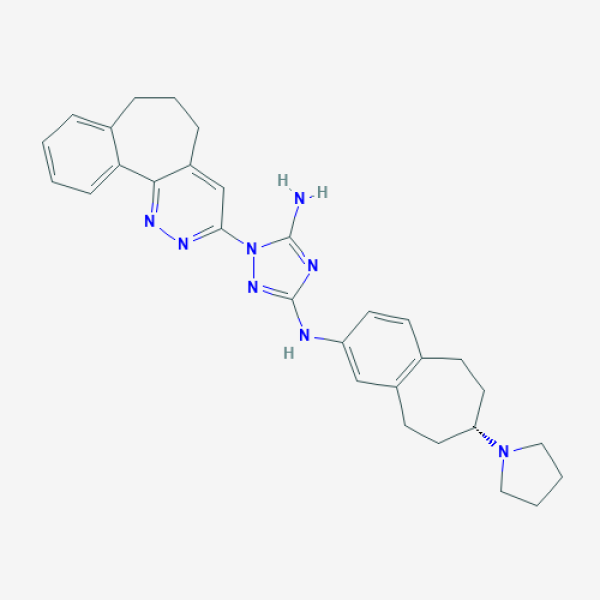
C1CCN(C1)[C@H]2CCC3=C(CC2)C=C(C=C3)NC4=NN(C(=N4)N)C5=NN=C6C(=C5)CCCC7=CC=CC=C76
Supporting references
| Link | Tested on | Impact factor | Notes | Publication date |
|---|---|---|---|---|
|
SARS-CoV-2 Mpro inhibitors: identification of anti-SARS-CoV-2 Mpro compounds from FDA approved drugs
3CLpro Small molecule In silico |
in silico | 3.22 | Predicted to inhibit the SARS-CoV-2 3C-like protease. |
Nov/05/2020 |
|
Drug repurposing screens reveal cell-type-specific entry pathways and FDA-approved drugs active against SARS-Cov-2
Small molecule In vitro Screening |
Huh7.5 cells; Calu-3 cells; primary normal human bronchial epithelial cells; iPSC-derived AT2 cells; SARS-CoV-2 strain USA WA1/2020 | 8.11 | Inhibited SARS-CoV-2 replication in Huh7.5 cells and also in Calu-3 cells with an SI of >3. |
Mar/23/2021 |
AI-suggested references
Clinical trials
| ID | Title | Status | Phase | Start date | Completion date |
|---|---|---|---|---|---|
| NCT04890509 | A Study of Bemcentinib for the Treatment of COVID-19 in Hospitalised Patients | Completed | Phase 2 | Oct/20/2020 | May/25/2021 |
|
|||||
