Arbidol
An indole derivative with antiviral properties.
General information
Arbidol is the brand name for an antiviral agent umifenovir, which is used for the treatment and prophylaxis of influenza and other respiratory infections in Russia and China. It is not approved by the FDA. Umifenovir inhibits membrane fusion, which prevents viral entry to the target cell, and therefore protects it from infection. The drug also stimulates the humoral immune response, induces interferon production, and stimulates the phagocytic function of macrophages. Recent studies indicate that umifenovir is effective at preventing entry of ebolavirus, human herpes virus 8, poliovirus and hepatitis B virus.
Regarding SARS-CoV-2, our AIM tool found the data that arbidol could help against SARS-CoV-2 by inhibiting its replication.
Arbidol therapy (200 mg tid for adults, no longer than 10 days) used to be one of the antiviral therapies of COVID-19 recommended by China's National Health Commission guidelines. It is NO longer recomended, however (Gui-Qiang et al., 2021).
Arbidol on DrugBank
Arbidol on PubChem
Arbidol on Wikipedia
Synonyms
Umifenovir
Marketed as
ARBIDOL; ARBIDOLE
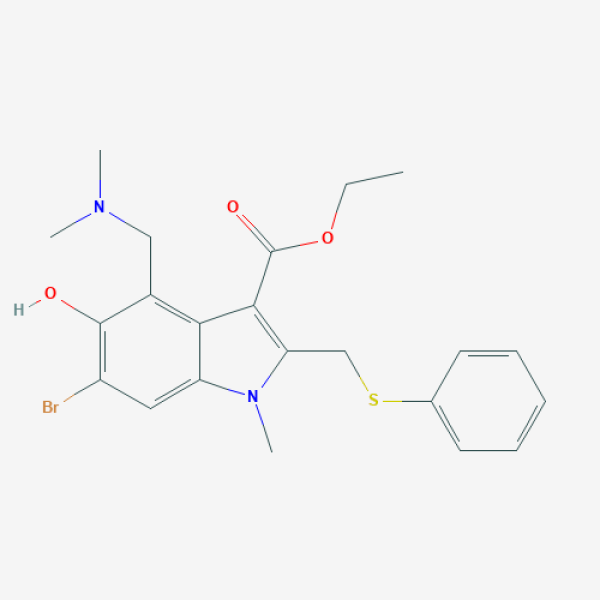
CCOC(=O)C1=C(N(C2=CC(=C(C(=C21)CN(C)C)O)Br)C)CSC3=CC=CC=C3
Supporting references
| Link | Tested on | Impact factor | Notes | Publication date |
|---|---|---|---|---|
|
CT Manifestations of Novel Coronavirus Pneumonia: A Case Report
|
Patient | Alpha interferon aerosol inhalation (dose for adults: five million U; add sterile water for injection, 2 mL, twice daily) with oral abidol (200 mg for adults, three times a day) |
Mar/06/2020 | |
|
Clinical characteristics and therapeutic procedure for four cases with 2019 novel coronavirus pneumonia receiving combined Chinese and Western medicine treatment.
|
Patients | combination of lopinavir/ritonavir (Kaletra®), arbidol, and Shufeng Jiedu Capsule (SFJDC, a traditional Chinese medicine) |
Mar/16/2020 | |
|
Favipiravir versus Arbidol for COVID-19: A Randomized Clinical Trial
|
Patients | Arbidol has comparable effects on clinical recovery rate of day 7 with favipiravir, but is less effective in improving the latency to cough relief and decreasing the duration of fever. |
Apr/08/2020 | |
|
Discovering drugs to treat coronavirus disease 2019 (COVID-19).
|
in silico | oral, 200 mg each time, 3 times/day |
Feb/22/2020 | |
|
The effect of Arbidol Hydrochloride on reducing mortality of Covid-19 patients: a retrospective study of real world date from three hospitals in Wuhan
|
Patients | even better in combination with oseltamivir |
Apr/17/2020 | |
|
No Clear Benefit to the Use of Corticosteroid as Treatment in Adult Patients with Coronavirus Disease 2019 : A Retrospective Cohort Study
|
Patients | in combination with ribavirin |
Apr/24/2020 | |
|
Prolonged SARS-CoV-2 Viral Shedding in Patients with COVID-19 was Associated with Delayed Initiation of Arbidol Treatment: a retrospective cohort study
Preprint Cohort study |
Patients | Early initiation of arbidol and arbidol combination with interferon is helpful |
Jun/10/2020 | |
|
Treatment with Arbidol and Moxifloxacin in Ordinary and Severe Adult Patients Infected with COVID-19
Severe severity Preprint |
Patients | in combination with moxifloxacin |
Jun/05/2020 | |
|
Discovery of Synergistic and Antagonistic Drug Combinations against SARS-CoV-2 In Vitro
Preprint In silico |
VERO E6 cell cultures | synergistic effect in combination with amodiaquine, nitazocanide, lopinavir and NCGC00411883-01 |
Jul/01/2020 | |
|
Identification of Potent and Safe Antiviral Therapeutic Candidates Against SARS-CoV-2
Small molecule In vitro Screening |
Vero cells | 5.09 | arbidol HCl |
Nov/25/2020 |
|
Effect of Arbidol (Umifenovir) on COVID-19: a randomized controlled trial
Small molecule Randomized controlled open trial |
Patients | 2.69 | Patients treated with Arbidol had significantly shorter length of hospital stay compared to lopinavir/ritonavir treatment and improved in various clinical, radiological, and laboratory parameters. There was insignificant difference in some parameters (e. g. time to fever relier, CRP). Sample size: 50 arbidol (hydroxychloroquine on day 1) + 50 lopinavir/ritonavir (hydroxychloroquine on day 1). Dosage: Treatment initiated with two doses of 400 mg hydroxychloroquine on day one; 200 mg of arbidol three times a day for 7 to 14 days. Endpoints: Hospital stay length and clinical improvement on day 7 (primary outcomes). |
Dec/14/2020 |
|
Efficacy of Early Combination Therapy With Lianhuaqingwen and Arbidol in Moderate and Severe COVID-19 Patients: A Retrospective Cohort Study
Severe severity Small molecule Moderate severity Cohort study |
Patients | 3.86 | Moderate (but not severe) patients treated with arbidol combined with <a href= |
Sep/18/2020 |
|
Screening, simulation, and optimization design of small molecule inhibitors of the SARS-CoV-2 spike glycoprotein
Spike protein Small molecule In silico |
in silico | 2.74 | Predicted to bind SARS-CoV-2 Spike protein. The drug's |
Jan/25/2021 |
|
Unraveling the mechanism of arbidol binding and inhibition of SARS-CoV-2: Insights from atomistic simulations
Spike protein ACE2 Small molecule In silico |
in silico | 3.26 | Predicted to bind the SARS-CoV-2 Spike protein RBD interface with the host's ACE2 receptor. |
Dec/31/2020 |
|
Beneficial effect of Arbidol in the management of COVID-19 infection
Severe severity Small molecule Critical severity Moderate severity Cohort study |
Patients | 4.83 | A higher rate of clinical improvement was observed in the treatment group compared to control. In a subgroup analysis, this outcome was observed for moderate to severe but not critical COVID-19 patients. The data were collected for infections that occurred in an earlier phase of the COVID-19 pandemic. Sample size: 228 + 24 control. Dosage: 200 mg three times a day. Primary outcome: (non)Improvement in the clinical status. |
Apr/03/2021 |
Clinical trials
| ID | Title | Status | Phase | Start date | Completion date |
|---|---|---|---|---|---|
| NCT04350684 | Umifenovir in Hospitalized COVID-19 Patients | Enrolling by invitation | Phase 4 | Apr/15/2020 | Apr/24/2020 |
|
|||||
| NCT04260594 | Clinical Study of Arbidol Hydrochloride Tablets in the Treatment of Pneumonia Caused by Novel Coronavirus | Completed | Phase 4 | Feb/08/2020 | Dec/30/2020 |
|
|||||
| NCT04476719 | The Bioequivalence Study of Umifenovir 200 mg Capsul (ATABAY, Turkey) Under Fasting Conditions | Active, not recruiting | Phase 1 | Jul/09/2020 | Aug/20/2020 |
|
|||||
| NCT04252885 | The Efficacy of Lopinavir Plus Ritonavir and Arbidol Against Novel Coronavirus Infection | Completed | Phase 4 | Jan/28/2020 | May/31/2020 |
|
|||||
| NCT04286503 | The Clinical Study of Carrimycin on Treatment Patients With COVID-19 | Unknown status | Phase 4 | Feb/23/2020 | Feb/28/2021 |
|
|||||
| NCT04273763 | Evaluating the Efficacy and Safety of Bromhexine Hydrochloride Tablets Combined With Standard Treatment/ Standard Treatment in Patients With Suspected and Mild Novel Coronavirus Pneumonia (COVID-19) | Active, not recruiting | Not Applicable | Feb/16/2020 | Jun/01/2020 |
|
|||||
