Aprepitant
A morpholine-based antiemetic.
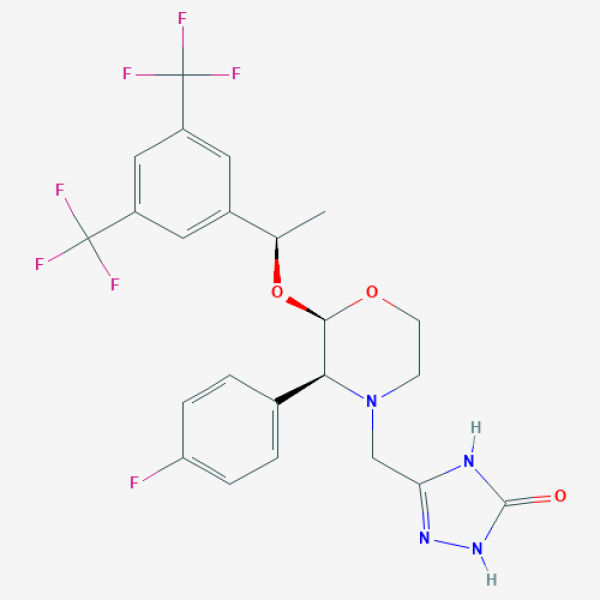
C[C@H](C1=CC(=CC(=C1)C(F)(F)F)C(F)(F)F)O[C@@H]2[C@@H](N(CCO2)CC3=NNC(=O)N3)C4=CC=C(C=C4)F
Supporting references
| Link | Tested on | Impact factor | Notes | Publication date |
|---|---|---|---|---|
|
Targeting SARS-CoV-2 Main Protease: A Computational Drug Repurposing Study
3CLpro Small molecule In silico |
in silico | 2.09 | Predicted to inhibit the SARS-CoV-2 3C-like protease. |
Sep/17/2020 |
AI-suggested references
Clinical trials
| ID | Title | Status | Phase | Start date | Completion date |
|---|---|---|---|---|---|
| NCT04470622 | Aprepitant Injectable Emulsion in Patients With COVID-19 (GUARDS-1) | Terminated | Phase 2 | Jul/20/2020 | Jun/03/2021 |
|
|||||
