Apilimod
A PIKfyve kinase inhibitor.
General information
Apilimod is a PIKfyve kinase inhibitor with immunomodulatory, anti-proliferatory and antiviral activities (Gayle et al., 2017; Nelson et al., 2017).
Apilimod on DrugBank
Apilimod on PubChem
Apilimod on Wikipedia
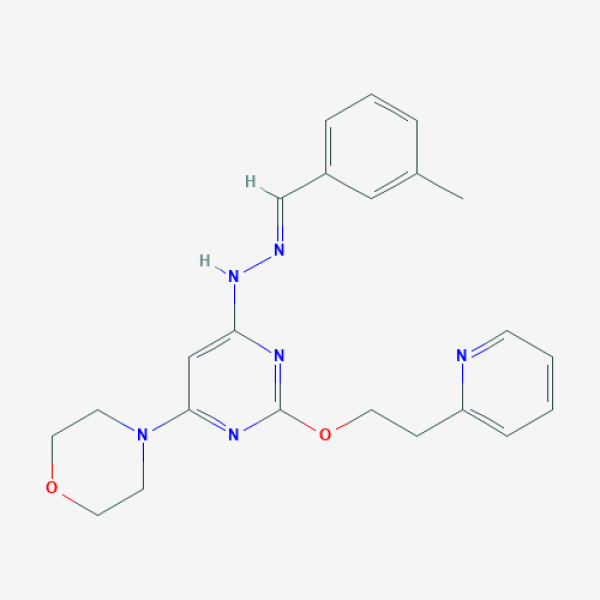
CC1=CC(=CC=C1)/C=N/NC2=CC(=NC(=N2)OCCC3=CC=CC=N3)N4CCOCC4
Supporting references
| Link | Tested on | Impact factor | Notes | Publication date |
|---|---|---|---|---|
|
Oral drug repositioning candidates and synergistic remdesivir combinations for the prophylaxis and treatment of COVID-19
Preprint In vitro Screening |
HeLa-ACE2 cells | Jun/16/2020 | ||
|
Discovery of SARS-CoV-2 antiviral drugs through large-scale compound repurposing
Small molecule In vitro Screening |
Vero E6 cells | 42.78 | Jul/24/2020 | |
|
Inhibition of PIKfyve kinase prevents infection by Zaire ebolavirus and SARS-CoV-2
Small molecule In vitro |
SVG-A cells; VERO E6 cells; SARS-CoV-2 strain 2019-nCoV/USA-WA1/2020 | 9.41 | Inhibits SARS-CoV-2 release into cell from endosomes in an in vitro model, likely through PIKfyve kinase inhibition and consequent cathepsin maturation impairment. Inhibits VERO E6 cells infection by authentic SARS-CoV-2. |
Aug/06/2020 |
AI-suggested references
Clinical trials
| ID | Title | Status | Phase | Start date | Completion date |
|---|---|---|---|---|---|
| NCT04446377 | A Study of LAM-002A for the Prevention of Progression of COVID-19 | Recruiting | Phase 2 | Jul/15/2020 | Apr/30/2021 |
|
|||||
