|
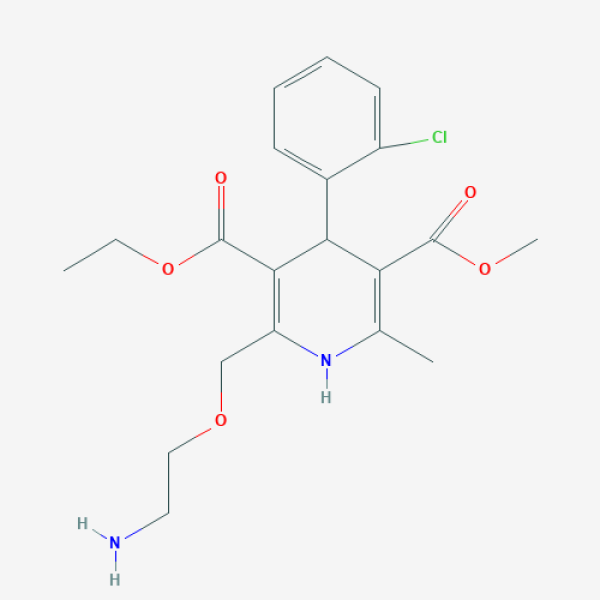
NCT04435028
|
Ketotifen: as a Cardioprotective Agent in Breast Cancer Patients Receiving Anthracycline-containing Chemotherapy |
Completed |
|
Jan/14/2019 |
Aug/13/2019 |
- Alternative id - 1890-1-2019
- Interventions - Drug: Ketotifen 1 MG
- Study type - Observational
- Study results - No Results Available
- Locations - Horus University, Damietta, Damiete Governonate, Egypt
- Study designs - Observational Model: Other|Time Perspective: Prospective
- Enrollment - 111
- Age - 30 Years to 60 Years (Adult)
- Outcome measures - prophylaxis effect of Ketotifen on patient's hearts during the treatment of anthracyclines
|
|
NCT04421534
|
Utility of Lactoferrin as an Adjunct Therapeutic Agent for COVID-19 |
Not yet recruiting |
Phase 2|Phase 3 |
Jun/01/2020 |
Nov/01/2020 |
- Alternative id - CUKA-001
- Interventions - Drug: Lactoferrin
- Study type - Interventional
- Study results - No Results Available
- Locations -
- Study designs - Allocation: Randomized|Intervention Model: Parallel Assignment|Masking: None (Open Label)|Primary Purpose: Treatment
- Enrollment - 150
- Age - 18 Years to 65 Years (Adult, Older Adult)
- Outcome measures - Time to clinical improvement|Rate of virological cure
|
|
NCT04251871
|
Treatment and Prevention of Traditional Chinese Medicines (TCMs) on COVID-19 Infection |
Recruiting |
Not Applicable |
Jan/22/2020 |
Jan/22/2021 |
- Alternative id - 2020001D
- Interventions - Drug: Conventional medicines (Oxygen therapy, alfa interferon via aerosol inhalation, and lopinavir/ritonavir) and Traditional Chinese Medicines (TCMs) granules|Drug: Conventional medicines (Oxygen therapy, alfa interferon via aerosol inhalation, and lopinavir/ritonavir)
- Study type - Interventional
- Study results - No Results Available
- Locations - The Fifth Medical Center, General Hospital of PLA, Beijing, Beijing, China
- Study designs - Allocation: Randomized|Intervention Model: Parallel Assignment|Masking: None (Open Label)|Primary Purpose: Treatment
- Enrollment - 150
- Age - 14 Years to 80 Years (Child, Adult, Older Adult)
- Outcome measures - The incidents of acute respiratory distress syndrome (ARDS) development|The time to fever resolution rate|Time to recovery of lung injury
|
|
NCT04934111
|
Safety and Immunogenicity of LNP-nCOV saRNA-02 Vaccine Against SARS-CoV-2, the Causative Agent of COVID-19 |
Not yet recruiting |
Phase 1 |
Sep/01/2021 |
Aug/01/2022 |
- Alternative id - COVAC Uganda
- Interventions - Drug: LNP-nCOV saRNA-02 Vaccine
- Study type - Interventional
- Study results - No Results Available
- Locations - MRC/UVRI & LSHTM Uganda Research Unit, Entebbe, Uganda
- Study designs - Allocation: N/A|Intervention Model: Single Group Assignment|Masking: None (Open Label)|Primary Purpose: Prevention
- Enrollment - 42
- Age - 18 Years to 45 Years (Adult)
- Outcome measures - Number of participants with solicited local injection site reactions|Number of participants with solicited systemic reactions starting within 7 days of administration of the vaccine|Number of participants with unsolicited adverse reactions (ARs) throughout the study|Number of participants with serious Adverse Events|Number of participants with unsolicited adverse events|The titer of serum neutralizing antibodies 2 weeks after the second vaccination in the SARS-CoV-2 pseudovirus-based neutralization assay|The titer of vaccine-induced serum IgG binding antibody responses to the SARS-CoV-2 S glycoprotein 2 weeks after the first and second vaccinations|Cell-mediated vaccine-induced immune responses measured by T- and B- cell ELISpot in study participants|Cell-mediated vaccine-induced immune responses measured by flow cytometry and intracellular cytokine staining in study participants|The profile of class and sub-class of antibody response|Laboratory markers of infection and infection-induced immunity|Incidence of thrombocytopenia of any grade confirmed on repeat testing if possible
|
|
NCT04350684
|
Umifenovir in Hospitalized COVID-19 Patients |
Enrolling by invitation |
Phase 4 |
Apr/15/2020 |
Apr/24/2020 |
- Alternative id - Umifenovir in COVID-19
- Interventions - Drug: Umifenovir|Drug: Interferon-β 1a|Drug: Lopinavir / Ritonavir|Drug: Single Dose of Hydroxychloroquine|Drug: Standards of Care
- Study type - Interventional
- Study results - No Results Available
- Locations - Loghman Hakim Hospital, Shahid Beheshti University of Medical Sciences and Health Services, Tehran, Iran, Islamic Republic of
- Study designs - Allocation: Randomized|Intervention Model: Parallel Assignment|Masking: Triple (Participant, Care Provider, Investigator)|Primary Purpose: Treatment
- Enrollment - 40
- Age - 18 Years and older (Adult, Older Adult)
- Outcome measures - Time to clinical improvement|Mortality|SpO2 Improvement|Incidence of new mechanical ventilation use|Duration of hospitalization|Cumulative incidence of serious adverse events
|
|
NCT04675086
|
Aralast NP With Antiviral Treatment and Standard of Care Versus Antiviral Treatment With Standard of Care in Hospitalized Patients With Pneumonia and COVID-19 Infection |
Withdrawn |
Phase 3 |
Jan/01/2021 |
Dec/01/2021 |
- Alternative id - CCR-2020-103188
- Interventions - Drug: alpha1-proteinase inhibitor|Drug: Antiviral Agents
- Study type - Interventional
- Study results - No Results Available
- Locations - Blessing Corporate Services, Inc, Hannibal, Missouri, United States
- Study designs - Allocation: Randomized|Intervention Model: Parallel Assignment|Masking: None (Open Label)|Primary Purpose: Treatment
- Enrollment - 0
- Age - 18 Years and older (Adult, Older Adult)
- Outcome measures - Duration of new non-invasive ventilation or high flow oxygen use (measured by days)|Clinical status on a 7-point ordinal scale (from 1=death to 7=not hospitalized|The percentage change in cytokine levels from screening through day 10, Day 17 and Day 24|The percentage change in oxygen requirements including PEEP and FiO2 from screening through day 10.|The percentage of subjects that required mechanical ventilation during the treatment period.|The percent of patients with a SOFA score between 0-6 during treatment period.|The percent of mortality during the treatment period.|Evaluate the need, dosage and duration of vasopressors (number of days and average daily dose).|Number of Days fever free (defined by temperature of <100°F (oral) for 24 hours)|To evaluate the average number of days in the ICU|To evaluate the average number of days in the hospital|To evaluate the number of days with a PO2/FiO2 <300 or other parameters decided on with oxygen|The risk of coagulopathy by measuring Prothrombin time & Partial Thromboplastin time|The risk of coagulopathy by measuring D-Dimer|The risk of coagulopathy by measuring Platelet Counts
|
|
NCT04427865
|
Utility of Lactoferrin as a Preventive Agent for Healthcare Workers Exposed to COVID-19 |
Not yet recruiting |
Phase 2|Phase 3 |
Jul/01/2020 |
Nov/01/2020 |
- Alternative id - CUKA-002
- Interventions - Drug: prophylactic lactoferrin daily
- Study type - Interventional
- Study results - No Results Available
- Locations -
- Study designs - Allocation: Randomized|Intervention Model: Parallel Assignment|Masking: None (Open Label)|Primary Purpose: Prevention
- Enrollment - 200
- Age - 18 Years to 65 Years (Adult, Older Adult)
- Outcome measures - Incidence of SARS-CoV-2|Severity of disease in confirmed infected participants
|
|
NCT04884750
|
Conversational Agent Vaccine Promotion RCT |
Not yet recruiting |
Not Applicable |
Jul/01/2022 |
Jan/31/2025 |
- Alternative id - IRB#18-07-02
- Interventions - Behavioral: ECA-ACE Vaccination Promotion Intervention
- Study type - Interventional
- Study results - No Results Available
- Locations -
- Study designs - Allocation: Randomized|Intervention Model: Factorial Assignment|Masking: Triple (Participant, Investigator, Outcomes Assessor)|Primary Purpose: Prevention
- Enrollment - 600
- Age - 18 Years and older (Adult, Older Adult)
- Outcome measures - Change of SARS-CoV-2 vaccination status|Change of Influenza vaccination status Influenza vaccination status|Change of Satisfaction Status|Stage of Change for Vaccination|Self-Efficacy for Vaccination|Decisional Balance for Vaccination|Knowledge
|
|
NCT04426084
|
Cardiovascular Risk Factors and Severe COVID-19. A Nationwide Registry-based Case-Control Study |
Active, not recruiting |
|
Mar/01/2020 |
Dec/31/2022 |
- Alternative id - 4
- Interventions - Other: Hypertension|Other: Diabetes type 2|Other: Obesity|Drug: Antihypertensive Agents|Drug: Statins (Cardiovascular Agents)
- Study type - Observational
- Study results - No Results Available
- Locations - Södersjukhuset, Stockholm, Sweden
- Study designs - Observational Model: Case-Control|Time Perspective: Other
- Enrollment - 22784
- Age - 18 Years and older (Adult, Older Adult)
- Outcome measures - Severe Covid-19|Severe Covid-19 with pulmonary embolism|CRRT(Continuous Renal Replacement Therapy)|ECMO (Extracorporeal Membrane Oxygenation )|ICU Mortality
|
|
NCT04341688
|
A Clinical Trial of Gargling Agents in Reducing Intraoral Viral Load Among COVID-19 Patients |
Not yet recruiting |
Not Applicable |
Dec/01/2021 |
Jul/31/2022 |
- Alternative id - 2020-Sur-ERC-4926
- Interventions - Drug: Gargle/Mouthwash
- Study type - Interventional
- Study results - No Results Available
- Locations -
- Study designs - Allocation: Randomized|Intervention Model: Parallel Assignment|Masking: Quadruple (Participant, Care Provider, Investigator, Outcomes Assessor)|Primary Purpose: Supportive Care
- Enrollment - 50
- Age - 18 Years to 65 Years (Adult, Older Adult)
- Outcome measures - Intraoral viral load|Salivary cytokine profile
|
|
NCT02735707
|
Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community- Acquired Pneumonia |
Recruiting |
Phase 3 |
Apr/11/2016 |
Dec/01/2025 |
- Alternative id - U1111-1189-1653|2015-002340-14|602525|16/631|APP1101719|158584
- Interventions - Drug: Ceftriaxone|Drug: Moxifloxacin or Levofloxacin|Drug: Piperacillin-tazobactam|Drug: Ceftaroline|Drug: Amoxicillin-clavulanate|Drug: Standard course macrolide|Drug: Extended course macrolide|Other: No systemic corticosteroid|Drug: Fixed-duration Hydrocortisone|Drug: Shock-dependent hydrocortisone|Drug: Fixed-duration higher dose Hydrocortisone|Other: No antiviral agent for influenza|Drug: Five-days oseltamivir|Drug: Ten-days oseltamivir|Other: No antiviral agent for COVID-19|Drug: Lopinavir / Ritonavir|Drug: Hydroxychloroquine|Drug: Hydroxychloroquine + lopinavir/ritonavir|Drug: Ivermectin|Other: No immune modulation for COVID-19|Drug: Interferon beta-1a|Drug: Anakinra|Drug: Tocilizumab|Drug: Sarilumab|Drug: Local standard venous thromboprophylaxis|Drug: Therapeutic anticoagulation|Drug: Conventional low dose thromboprophylaxis|Drug: Intermediate dose thromboprophylaxis|Drug: Continuation of therapeutic dose anticoagulation|Other: No immunoglobulin|Biological: Convalescent plasma|Biological: Delayed administration of convalescent plasma|Other: No vitamin C|Drug: Vitamin C|Other: No antiplatelet|Drug: Aspirin|Drug: P2Y12 inhibitor|Other: No simvastatin|Drug: Simvastatin|Other: Placebo|Drug: Eritoran|Drug: Apremilast|Procedure: Clinician-preferred mechanical ventilation strategy|Procedure: Protocolised mechanical ventilation strategy|Other: No renin-angiotensin system inhibitor|Drug: Angiotensin converting enzyme inhibitor|Drug: Angiotensin Receptor Blockers|Drug: ARB + DMX-200|Other: No cysteamine|Drug: Cysteamine
- Study type - Interventional
- Study results - No Results Available
- Locations - University of Florida, Jacksonville, Florida, United States|Augusta University, Augusta, Georgia, United States|University of Illinois Health, Chicago, Illinois, United States|Tulane Medical Center, New Orleans, Louisiana, United States|University of Michigan, Ann Arbor, Michigan, United States|Memorial Sloan Kettering Cancer Center, New York, New York, United States|Wake Forest Baptist Health, Winston-Salem, North Carolina, United States|The Ohio State University Wexner Medical Center, Columbus, Ohio, United States|Oregon Health and Science University, Portland, Oregon, United States|University of Pittsburgh Medical Centre, Pittsburgh, Pennsylvania, United States|Brown University - Rhode Island Hospital, Providence, Rhode Island, United States|Canberra Hospital, Canberra, Australian Capital Territory, Australia|Bankstown-Lidcombe Hospital, Bankstown, New South Wales, Australia|Blacktown Hospital, Blacktown, New South Wales, Australia|Campbelltown Hospital, Campbelltown, New South Wales, Australia|Sutherland Hospital, Caringbah, New South Wales, Australia|Concord Hospital, Concord, New South Wales, Australia|Dubbo Base Hospital, Dubbo, New South Wales, Australia|Northern Beaches Hospital, Frenchs Forest, New South Wales, Australia|Nepean Hospital, Kingswood, New South Wales, Australia|St. George Hospital, Kogarah, New South Wales, Australia|Liverpool Hospital, Liverpool, New South Wales, Australia|John Hunter Hospital, Newcastle, New South Wales, Australia|Orange Health Service, Orange, New South Wales, Australia|St Vincent's Hospital Sydney, Sydney, New South Wales, Australia|Prince of Wales Hospital, Sydney, New South Wales, Australia|Royal Prince Alfred Hospital, Sydney, New South Wales, Australia|Royal North Shore Hospital, Sydney, New South Wales, Australia|Wollongong Hospital, Sydney, New South Wales, Australia|Wagga Wagga Base Hospital, Wagga Wagga, New South Wales, Australia|Westmead Hospital, Westmead, New South Wales, Australia|Royal Darwin Hospital,, Darwin, Northern Territory, Australia|Sunshine Coast University Hospital, Birtinya, Queensland, Australia|The Prince Charles Hospital, Brisbane, Queensland, Australia|Mater Hospital Brisbane, Brisbane, Queensland, Australia|Princess Alexandra Hospital, Brisbane, Queensland, Australia|Caboolture Hospital, Caboolture, Queensland, Australia|Queen Elizabeth II Jubilee Hospital, Coopers Plains, Queensland, Australia|Logan Hospital, Logan, Queensland, Australia|Redcliffe Hospital, Redcliffe, Queensland, Australia|Rockhampton Hospital, Rockhampton, Queensland, Australia|Gold Coast University Hospital, Southport, Queensland, Australia|Toowoomba Hospital, Toowoomba, Queensland, Australia|Townsville Hospital, Townsville, Queensland, Australia|Royal Adelaide Hospital, Adelaide, South Australia, Australia|The Queen Elizabeth Hospital, Adelaide, South Australia, Australia|Lyell McEwin Hospital, Adelaide, South Australia, Australia|Flinders Medical Centre, Bedford Park, South Australia, Australia|Launceston Hospital, Launceston, Tasmania, Australia|Ballarat Base Hospital, Ballarat, Victoria, Australia|Bendigo Hospital, Bendigo, Victoria, Australia|Casey Hospital, Berwick, Victoria, Australia|Box Hill Hospital, Box Hill, Victoria, Australia|Monash Medical Centre, Clayton, Victoria, Australia|Dandenong Hospital, Dandenong, Victoria, Australia|Angliss Hospital, Ferntree Gully, Victoria, Australia|Footscray Hospital, Footscray, Victoria, Australia|University Hosptial Geelong, Geelong, Victoria, Australia|The Alfred Hospital, Melbourne, Victoria, Australia|Royal Melbourne Hospital, Melbourne, Victoria, Australia|St Vincent's Hospital Melbourne, Melbourne, Victoria, Australia|Maroondah Hospital, Ringwood East, Victoria, Australia|Sunshine Hospital, Sunshine, Victoria, Australia|Werribee Mercy Hospital, Werribee, Victoria, Australia|St John of God Hospital Midland, Midland, Western Australia, Australia|St John of God Hospital Murdoch, Murdoch, Western Australia, Australia|Royal Perth Hospital, Perth, Western Australia, Australia|Sir Charles Gairdner Hospital, Perth, Western Australia, Australia|Fiona Stanley Hospital, Perth, Western Australia, Australia|St John of God Subiaco, Subiaco, Western Australia, Australia|AZ Sint-Jan, Brugge, Belgium|CHU de Charleroi - Hôpital Civil Marie Curie, Charleroi, Belgium|Universitair Ziekenhuis Antwerp, Edegem, Belgium|Universitair Ziekenhuis Gent, Gent, Belgium|Foothills Medical Centre, Calgary, Alberta, Canada|Peter Lougheed Centre, Calgary, Alberta, Canada|Rockyview General Hospital, Calgary, Alberta, Canada|South Health Campus, Calgary, Alberta, Canada|Royal Alexandra Hospital, Alberta, Edmonton, Alberta, Canada|University of Alberta Hospital, Edmonton, Alberta, Canada|Surrey Memorial Hospital, Surrey, British Columbia, Canada|St Boniface General Hospital, Winnipeg, Manitoba, Canada|Health Sciences Centre Winnipeg, Winnipeg, Manitoba, Canada|Grace Hospital, Winnipeg, Manitoba, Canada|Dr. Everett Chalmers Regional Hospital, Fredericton, New Brunswick, Canada|The Moncton Hospital, Fredericton, New Brunswick, Canada|The Saint John General Hospital, Fredericton, New Brunswick, Canada|William Osler Health System, Brampton, Ontario, Canada|Brantford General Hospital, Brantford, Ontario, Canada|Hamilton general Hospital, Hamilton, Ontario, Canada|Juravinski Hospital, Hamilton, Ontario, Canada|St. Joseph's Healthcare Hamilton, Hamilton, Ontario, Canada|Kingston Health Sciences Centre, Kingston, Ontario, Canada|Grand River Hospital, Kitchener, Ontario, Canada|St Mary's General Hospital, Kitchener, Ontario, Canada|The Ottawa Hospital, Ottawa, Ontario, Canada|Niagara Health, Saint Catharines, Ontario, Canada|Thunder Bay General Hospital, Thunder Bay, Ontario, Canada|Sunnybrook Health Sciences Centre, Toronto, Ontario, Canada|St. Michael's Hospital Unity Health Toronto, Toronto, Ontario, Canada|Mount Sinai Hospital, Toronto, Ontario, Canada|Toronto General Hospital, Toronto, Ontario, Canada|Toronto Western Hospital, Toronto, Ontario, Canada|St Joseph's Health Centre, Toronto, Ontario, Canada|CIUSS Chaudieres-Appalaches (Levis), Lévis, Quebec, Canada|Hospital Maisonneuve-Rosemont, Montréal, Quebec, Canada|Hôpital Fleury, Montréal, Quebec, Canada|Centre Hospitalier de l'Universite de Montreal, Montréal, Quebec, Canada|McGill University Health Centre, Montréal, Quebec, Canada|Hopital du Sacre-Coeur de Montreal, Montréal, Quebec, Canada|CHU de Québec - Université Laval, Québec, Quebec, Canada|IUCPQ-UL, Québec, Quebec, Canada|Centre Hospitalier de l'Université de Sherbrooke, Sherbrooke, Quebec, Canada|Regina General Hospital, Saskatoon, Saskatchewan, Canada|Universidad de La Sabana, Chía, Cundinamarca, Colombia|General County Hospital Požega, Požega, Croatia|University Hospital Centre Zagreb, Zagreb, Croatia|University Hospital for Infectious Diseases, Zagreb, Croatia|Charité - Universitätsmedizin Berlin - Infektiologie und Pneumologie, Berlin, Germany|Charité - Universitätsmedizin Berlin - Nephrologie, Berlin, Germany|Vivantes Klinikum Neukölln, Berlin, Germany|Universitätsklinikum Köln, Cologne, Germany|Universitätsklinikum Frankfurt, Frankfurt, Germany|University Medical Center Hamburg-Eppendorf (UKE), Hamburg, Germany|Medizinische Hochschule Hannover, Hannover, Germany|Universitätsklinikum Jena, Jena, Germany|Universitätsklinikum Leipzig, Leipzig, Germany|Universitäts Klinikum Tübingen, Tübingen, Germany|Universitätsklinikum Würzburg, Würzburg, Germany|Jósa András County Hospital, Nyíregyháza, Hungary|Csolnoky Ferenc Kórház - Veszprem County Hospital, Veszprém, Hungary|Almási Balogh Pál Kórház, Ózd, Hungary|Apollo Main Hospital, Chennai, Tamil Nadu, India|Apollo First Med Hospital, Chennai, Tamil Nadu, India|Apollo Vanagaram Hospital, Chennai, Tamil Nadu, India|Apollo Speciality Hospital - OMR, Chennai, Tamil Nadu, India|Beaumont Hospital, Dublin, Ireland|St. Vincent's University Hospital, Dublin, Ireland|University Hospital Galway, Galway, Ireland|St Marianna University School of Medicine, Kawasaki, Kanagawa, Japan|Yokohama City University Hospital, Yokohama, Kanagawa, Japan|St. Marianna University Yokohama City Seibu Hospital, Yokohama, Kanagawa, Japan|Saiseikai Kumamoto Hospital, Minami, Kumamoto, Japan|Osaka City General Hospital, Osaka, Japan|Nerima Hikarigaoka Hospital, Tokyo, Japan|Tokyo Metropolitan Bokutoh Hospital, Tokyo, Japan|Itabashi Chuo Medical Center, Tokyo, Japan|Tokyo bay Urayasu-Ichikawa Medical Center, Tokyo, Japan|Wakayama Medical University, Wakayama, Japan|Chitwan Medical College, Bharatpur, Nepal|Grande International Hospital, Kathmandu, Nepal|Hospital for Advanced Medicine and Surgery (HAMS), Kathmandu, Nepal|Nepal Mediciti, Kathmandu, Nepal|Meander Medisch Centrum, Amersfoort, Netherlands|Jeroen Bosch Ziekenhuis, Den Bosch, Netherlands|Martini Hospital Groningen, Groningen, Netherlands|University Medical Center Groningen, Groningen, Netherlands|Leiden University Medical Center, Leiden, Netherlands|Canisius Wilhelmina Ziekenhuis, Nijmegen, Netherlands|Radboud University Medical Center, Nijmegen, Netherlands|University Medical Center Utrecht, Utrecht, Netherlands|North Shore Hospital, Auckland, New Zealand|CVICU, Auckland City Hospital, Auckland, New Zealand|DCCM, Auckland City Hospital, Auckland, New Zealand|Middlemore Hospital, Auckland, New Zealand|Christchurch Hospital, Christchurch, New Zealand|Waikato Hospital, Hamilton, New Zealand|Taranaki Base Hospital, New Plymouth, New Zealand|Rotorua Hospital, Rotorua, New Zealand|Tauranga Hospital, Tauranga, New Zealand|Wellington Regional Hospital, Wellington, New Zealand|Whangarei Hospital, Whangarei, New Zealand|Ziauddin University Hospital Clifton Campus, Karachi, Sindh, Pakistan|Abbasi Shaheed Hospital, Karachi, Sindh, Pakistan|National Institute of Cardiovascular Diseases, Karachi, Karachi, Sindh, Pakistan|South City Hospital, Karachi, Karachi, Sindh, Pakistan|Ziauddin University North Nazimabad Campus, Karachi, Sindh, Pakistan|Centro Hospitalar do Medio Tejo, Abrantes, Portugal|Hospital Lusíadas Lisbon, Lisboa, Portugal|Clinical Hospital of Infectious and Tropical Diseases "Dr. Victor Babes", Bucharest, Romania|King Abdulaziz Medical City, Riyadh, Saudi Arabia|Institut Hospital del Mar d'Investigacions Mèdiques, Barcelona, Spain|Hospital Universitario Reina Sofia, Córdoba, Spain|Basildon Hospital, Basildon, England, United Kingdom|Basingstoke and North Hampshire Hospital, Basingstoke, England, United Kingdom|Royal United Hospital, Bath, Bath, England, United Kingdom|Queen Elizabeth Hospital Birmingham, Birmingham, England, United Kingdom|Birmingham City Hospital, Birmingham, England, United Kingdom|Blackburn Hospital, Blackburn, England, United Kingdom|Pilgrim's Hospital, Boston, England, United Kingdom|Royal Bournemouth Hospital, Bournemouth, England, United Kingdom|Royal Sussex County Hospital, Brighton, England, United Kingdom|Southmead Hospital, Bristol, England, United Kingdom|Bristol Royal Hospital, Bristol, England, United Kingdom|Queen's Hospital, Burton, Burton on Trent, England, United Kingdom|Royal Papworth Hospital, Cambridge, England, United Kingdom|Addenbrookes Hospital, Cambridge, England, United Kingdom|Cumberland Royal Infirmary, Carlisle, England, United Kingdom|Ashford & St Peters Hospital Trust, Chertsey, England, United Kingdom|Chesterfield Royal Hospital, Chesterfield, England, United Kingdom|Countess of Chester Hospital, Chester, England, United Kingdom|Colchester Hospital, Colchester, England, United Kingdom|University Hospital Coventry, Coventry, England, United Kingdom|North Manchester General Hospital, Crumpsall, England, United Kingdom|Darlington Memorial Hospital, Darlington, England, United Kingdom|Darent Valley Hospital, Dartford, England, United Kingdom|Russells Hall Hospital, Dudley, England, United Kingdom|University Hospital of North Durham, Durham, England, United Kingdom|Royal Devon and Exeter Hospital, Exeter, England, United Kingdom|Frimley Park Hospital, Frimley, England, United Kingdom|Queen Elizabeth Hospital, Gateshead, England, United Kingdom|Medway Maritime Hospital, Gillingham, England, United Kingdom|James Paget Kings Lynn Hospital, Great Yarmouth, England, United Kingdom|Royal Surrey County Hospital, Guildford, England, United Kingdom|Northwick Park Hospital, Harrow, England, United Kingdom|Hereford County Hospital, Hereford, England, United Kingdom|Barnet Hospital, High Barnet, England, United Kingdom|Huddersfield Hospital, Huddersfield, England, United Kingdom|King George Hospital, Ilford, England, United Kingdom|Ipswich Hospital, Ipswich, England, United Kingdom|Kettering Hospital, Kettering, England, United Kingdom|Leeds Teaching Hospitals NHS Trust, Leeds, England, United Kingdom|Leicester Royal Infirmary, Leicester, England, United Kingdom|Glenfield Hospital, Leicester, England, United Kingdom|Lincoln County Hospital, Lincoln, England, United Kingdom|Liverpool Heart and Chest Hospital, Liverpool, England, United Kingdom|Alder Hey Hospital, Liverpool, England, United Kingdom|Royal Liverpool Hospital, Liverpool, England, United Kingdom|University Hospital Aintree, Liverpool, England, United Kingdom|Croydon University Hospital, London, England, United Kingdom|Royal London Hospital, London, England, United Kingdom|Whipps Cross Hospital, London, England, United Kingdom|Newham Hospital, London, England, United Kingdom|St Barts Hosptial, London, England, United Kingdom|North Middlesex Hospital, London, England, United Kingdom|Royal Free Hospital, London, England, United Kingdom|St Thomas' Hospital, London, England, United Kingdom|Guy's Hospital, London, England, United Kingdom|King's College Hospital, London, England, United Kingdom|St George's Hospital, London, England, United Kingdom|Royal Marsden Hospital, London, England, United Kingdom|Ryal Brompton, London, England, United Kingdom|Hammersmith Hospital, London, England, United Kingdom|St Mary's Hospital, London, England, United Kingdom|Charing Cross Hospital, London, England, United Kingdom|Luton and Dunstable University Hospital, Luton, England, United Kingdom|Maidstone Hospital - Maidstone and Tunbridge Wells NHS Trust, Maidstone, England, United Kingdom|Manchester Royal Infirmary, Manchester, England, United Kingdom|The Christie Hospital, Manchester, England, United Kingdom|Wythenshawe Hospital, Manchester, England, United Kingdom|Queen Elizabeth Hospital, Woolwich, Margate, England, United Kingdom|The James Cook University Hospital, Middlesbrough, England, United Kingdom|Milton Keynes University Hospital, Milton Keynes, England, United Kingdom|Royal Victoria Infirmary, Newcastle, Newcastle, England, United Kingdom|Newcastle Freeman Hospital, Newcastle, England, United Kingdom|Northampton General Hospital, Northampton, England, United Kingdom|Norfolk and Norwich University Hospital, Norwich, England, United Kingdom|City Hospital Nottingham, Nottingham, England, United Kingdom|Queen's Medical Centre - Nottingham University Hospitals NHS Trust, Nottingham, England, United Kingdom|George Eliot Hospital, Nuneaton, England, United Kingdom|Royal Oldham Hospital, Oldham, England, United Kingdom|Princess Royal University Hospital, Orpington, England, United Kingdom|John Radcliffe Hospital, Oxford, England, United Kingdom|Derriford Hospital, Plymouth, England, United Kingdom|Poole Hospital NHS Foundation Trust, Poole, England, United Kingdom|Queen Alexandra Hospital - Portsmouth Hospitals NHS Trust, Portsmouth, England, United Kingdom|Whiston Hospital, Prescot, England, United Kingdom|Royal Preston Hospital, Preston, England, United Kingdom|Royal Berkshire Hospital, Reading, England, United Kingdom|Alexandra Hospital, Redditch, Redditch, England, United Kingdom|Queen's Hospital Romford, Romford, England, United Kingdom|Rotherham General Hospital, Rotherham, England, United Kingdom|Salford Royal Hospital, Salford, England, United Kingdom|Salisbury District Hospital, Salisbury, England, United Kingdom|Royal Hallamshire Hospital, Sheffield, England, United Kingdom|Northern General Hospital, Sheffield, England, United Kingdom|Wexham Park Hospital, Slough, England, United Kingdom|South Tyneside District Hospital, South Shields, England, United Kingdom|Southampton General Hospital, Southampton, England, United Kingdom|Stepping Hill Hospital, Stockport, England, United Kingdom|University Hospital of North Tees, Stockton-on-Tees, England, United Kingdom|Royal Stoke University Hospital, Stoke-on-Trent, England, United Kingdom|Sunderland Hospital, Sunderland, England, United Kingdom|King's Mill Hospital, Sutton In Ashfield, England, United Kingdom|Great Western Hospital, Swindon, England, United Kingdom|Western General Hospital, Swindon, England, United Kingdom|Musgrove Park Hospital, Taunton, England, United Kingdom|Torbay and South Devon Hospital, Torquay, England, United Kingdom|Royal Cornwall Hospital, Truro, England, United Kingdom|Tunbridge Wells Hospital - Maidstone and Tunbridge Wells NHS Trust, Tunbridge Wells, England, United Kingdom|Harefield Hospital, Uxbridge, England, United Kingdom|Watford General Hospital, Watford, England, United Kingdom|Southend University Hospital, Westcliff-on-Sea, England, United Kingdom|West Cumberland Hospital, Whitehaven, England, United Kingdom|Royal Albert Edward Infirmary, Wigan, England, United Kingdom|Royal Hampshire Hospital, Winchester, England, United Kingdom|Arrow Park Hospital, Wirral, England, United Kingdom|New Cross Hospital, Wolverhampton, England, United Kingdom|Worcester Royal Hospital, Worcester, England, United Kingdom|York Hospital, York, England, United Kingdom|York Hospital, York, England, United Kingdom|Antrim Area Hospital, Antrim, Northern Ireland, United Kingdom|Royal Victoria Hospital, Belfast, Belfast, Northern Ireland, United Kingdom|Mater Hospital, Belfast, Northern Ireland, United Kingdom|Belfast City Hospital, Belfast, Northern Ireland, United Kingdom|Altnagelvin Hospital, Derry, Northern Ireland, United Kingdom|Aberdeen Royal Infirmary, Aberdeen, Scotland, United Kingdom|Ninewells Hospital, Dundee, Scotland, United Kingdom|Royal Infirmary of Edinburgh, Edinburgh, Scotland, United Kingdom|Glasgow Royal Infirmary, Glasgow, Scotland, United Kingdom|Queen Elizabeth University Hospital, Glasgow, Glasgow, Scotland, United Kingdom|Royal Alexandra Hospital, Glasgow, Paisley, Scotland, United Kingdom|Neville Hall Hospital, Abergavenny, Wales, United Kingdom|Glan Clywd Hospital, Bodelwyddan, Wales, United Kingdom|Princess of Wales Hospital, Bridgend, Wales, United Kingdom|University Hospital of Wales, Cardiff, Wales, United Kingdom|Glangwilli Hospital, Carmarthen, Wales, United Kingdom|Grange University Hospital, Cwmbran, Wales, United Kingdom|Royal Gwent Hospital, Newport, Wales, United Kingdom|Royal Glamorgan Hospital, Pontyclun, Wales, United Kingdom|Morriston Hospital, Swansea, Wales, United Kingdom|Wrexham Maelor Hospital, Wrexham, Wales, United Kingdom
- Study designs - Allocation: Randomized|Intervention Model: Factorial Assignment|Masking: None (Open Label)|Primary Purpose: Treatment
- Enrollment - 10000
- Age - 18 Years and older (Adult, Older Adult)
- Outcome measures - All-cause mortality|Days alive and not receiving organ support in ICU|ICU Mortality|ICU length of stay|Hospital length of stay|Ventilator free days|Organ failure free days|Health-related Quality of life assessment|Proportion of intubated patients who receive a tracheostomy|Destination at time of hospital discharge|Readmission to the index ICU during the index hospitalization|World Health Organisation 8-point ordinal scale outcome
|
|
NCT04738045
|
Comparison of Remdesivir Versus Lopinavir/ Ritonavir and Remdesivir Combination in COVID-19 Patients |
Recruiting |
Phase 4 |
Nov/01/2020 |
Apr/01/2021 |
- Alternative id - REC-H-PhBSU-21001
- Interventions - Drug: Remdesivir|Drug: Lopinavir/ Ritonavir and Remdesivir combination
- Study type - Interventional
- Study results - No Results Available
- Locations - Beni-suef University, Banī Suwayf, Egypt
- Study designs - Allocation: Non-Randomized|Intervention Model: Parallel Assignment|Masking: None (Open Label)|Primary Purpose: Treatment
- Enrollment - 90
- Age - 18 Years to 80 Years (Adult, Older Adult)
- Outcome measures - Proportion of cured patients in the interventional group versus the proportion of cured patients in the control group|Monitoring of adverse events.
|
|
NCT04521400
|
the Investigation Into Beneficial Effects of High-dose Interferon Beta 1-a, Compared to Low-dose Interferon Beta 1-a in Moderate to Severe Covid-19 |
Not yet recruiting |
Phase 2 |
Aug/20/2020 |
Sep/11/2020 |
- Alternative id - Interferon in COVID
- Interventions - Drug: High dose Interferon-beta 1a|Drug: Lopinavir/Ritonavir|Drug: Low dose Interferon-beta 1a
- Study type - Interventional
- Study results - No Results Available
- Locations -
- Study designs - Allocation: Randomized|Intervention Model: Parallel Assignment|Masking: None (Open Label)|Primary Purpose: Treatment
- Enrollment - 100
- Age - 18 Years to 100 Years (Adult, Older Adult)
- Outcome measures - Time to clinical improvement|Mortality|SpO2 Improvement|Incidence of new mechanical ventilation use|Duration of hospitalization|Cumulative incidence of serious adverse events
|
|
NCT04351724
|
Austrian CoronaVirus Adaptive Clinical Trial (COVID-19) |
Recruiting |
Phase 2|Phase 3 |
Apr/16/2020 |
Mar/31/2022 |
- Alternative id - ACOVACT
- Interventions - Drug: Chloroquine or Hydroxychloroquine|Drug: Lopinavir/Ritonavir|Other: Best standard of care|Drug: Rivaroxaban|Drug: Thromboprophylaxis|Drug: Candesartan|Drug: non-RAS blocking antihypertensives|Drug: Remdesivir|Drug: Asunercept 400mg|Drug: Asunercept 100mg|Drug: Asunercept 25mg|Drug: Pentaglobin
- Study type - Interventional
- Study results - No Results Available
- Locations - Medical University of Innsbruck, Innsbruck, Tirol, Austria|Medical University of Graz, Graz, Austria|Kepler University Hospital, Linz, Austria|Medical University of Vienna, Vienna, Austria|Wilhelminenspital, Vienna, Austria|SMZ Süd Kaiser Franz Josef Spital, Vienna, Austria|KH Hietzing, Vienna, Austria|SMZ Baumgartner Höhe Otto Wagner Spital, Vienna, Austria|SMZ Ost Donauspital, Vienna, Austria
- Study designs - Allocation: Randomized|Intervention Model: Parallel Assignment|Masking: None (Open Label)|Primary Purpose: Treatment
- Enrollment - 500
- Age - 18 Years to 99 Years (Adult, Older Adult)
- Outcome measures - sustained improvement (>48h) of one point on the WHO Scale|Time to improvement on WHO Scale|Mean change in the ranking on an ordinal scale from baseline|time to discharge or a National Early Warning Score (NEWS) ≤2 (maintained for 24h), whichever occurs first|change from baseline in National Early Warning Score (NEWS)|Oxygenation free days|Incidence of new oxygen use during the trial|duration of oxygen use during the trial|Ventilator free days until day 29|Incidence of new mechanical ventilation use during the trial|duration of mechanical ventilation use during the trial|Viral load/viral clearance|Duration of Hospitalization|Mortality|Obesity - mortality|Obesity - duration of hospitalization|Obesity - ICU admission|Obesity - new oxygen use|Drug-drug interactions with lopinavir/ritonavir|Renin Angiotensin System (RAS) fingerprint|SpO2/FiO2 ratio|paO/FiO2 ratio|modified Sequential Organ Failure Assessment|C-reactive protein|Interleukin-6|procalcitonin|IgM Concentrations|IgA Concentrations|differential blood counts
|
|
NCT04376814
|
Favipiravir Plus Hydroxychloroquine and Lopinavir/Ritonavir Plus Hydroxychloroquine in COVID-19 |
Completed |
Not Applicable |
Mar/29/2020 |
May/25/2020 |
- Alternative id - IR.BMSU.REC.1399.017
- Interventions - Drug: Favipiravir|Drug: Hydroxychloroquine|Drug: Lopinavir / Ritonavir
- Study type - Interventional
- Study results - No Results Available
- Locations - Mohammad Sadegh Bagheri Baghdasht, Tehran, Iran, Islamic Republic of
- Study designs - Allocation: Non-Randomized|Intervention Model: Parallel Assignment|Masking: None (Open Label)|Primary Purpose: Treatment
- Enrollment - 40
- Age - 16 Years to 100 Years (Child, Adult, Older Adult)
- Outcome measures - Mortality|long of hospitalization|Laboratory Treatment Response (Blood cell count)|Laboratory Treatment Response (CRP )|Dyspnea|Oxygen saturation without supplemental oxygen.|Oxygen therapy
|
|
NCT04466241
|
Combination Therapies to Reduce Carriage of SARS-Cov-2 and Improve Outcome of COVID-19 in Ivory Coast: a Phase Randomized IIb Trial |
Recruiting |
Phase 2|Phase 3 |
Nov/27/2020 |
Mar/26/2021 |
- Alternative id - ANRS COV01 INTENSE COV
- Interventions - Drug: Lopinavir/Ritonavir 200 MG-50 MG Oral Tablet|Drug: Telmisartan 40Mg Oral Tablet|Drug: Atorvastatin 20 Mg Oral Tablet
- Study type - Interventional
- Study results - No Results Available
- Locations - Service des Maladies Infectieuses et Tropicales, Centre Hospitalier et Universitaire (CHU) Treichville, Abidjan, Côte D'Ivoire|Centre de Traitement des Maladies Infectieuses (CTMI), CHU de Yopougon, Abidjan, Côte D'Ivoire
- Study designs - Allocation: Randomized|Intervention Model: Parallel Assignment|Masking: None (Open Label)|Primary Purpose: Treatment
- Enrollment - 294
- Age - 18 Years and older (Adult, Older Adult)
- Outcome measures - Proportion of patients with undetectable nasopharyngeal swab SARS-CoV-2 PCR and C-reactive protein (CRP) < 27 mg/L at Day 11|Proportion of patients with clinical improvement on the 7-point ordinal scale at Day 11 and Day 28|Kinetics of SARS-CoV-2 viral load|Death rate at Day 11 and Day 28|All causes of death and Acute respiratory distress syndrome (ARDS) at Day 28|Time to hospital discharge|Duration of oxygen supplementation|Prevalence of grade III or IV adverse events|Residual concentration of lopinavir, telmisartan and atorvastatin|Evolution of inflammatory and immunological markers (CRP, fibrinogen, ferritin, d-dimer, dosing of IgG, IgA, IgM; TCD4, CD8, B lymphocytes, NK lymphocytes; naïve/memory T lymphocytes)|Evolution of endothelial activation markers (VEGF and soluble VEGF receptor,VE-cadherin, PECAM/CD31, CD42 and angiopoietin-2)|Proportion of patients with good results according to HIV status|Number of contact cases infected by COVID-19 at Day 28
|
|
NCT04453501
|
Anti Infective Agents Impact in COVID-19 Pneumonia |
Completed |
|
Mar/02/2020 |
Apr/25/2020 |
- Alternative id - AZITHROVID|INDS
- Interventions - Drug: favorable outcome
- Study type - Observational
- Study results - No Results Available
- Locations - Benjamin Davido, Garches, France
- Study designs - Observational Model: Cohort|Time Perspective: Retrospective
- Enrollment - 132
- Age - 18 Years and older (Adult, Older Adult)
- Outcome measures - Favorable outcome|Risk factors 1|Risk factors 2|Interest of anti-infective agents
|
|
NCT04381871
|
Potential Role of Gum Arabic as Immunomodulatory Agent Among COVID 19 Patients |
Not yet recruiting |
Phase 2|Phase 3 |
Jun/01/2020 |
Sep/01/2020 |
- Alternative id - GA& COVID19
- Interventions - Dietary Supplement: Acacia Senegal|Dietary Supplement: Pectin
- Study type - Interventional
- Study results - No Results Available
- Locations - Omdurman Teaching Hospital, Khartoum, Omdurman, Sudan|Jabra Hospital,, Khartoum, Sudan
- Study designs - Allocation: Randomized|Intervention Model: Parallel Assignment|Masking: Quadruple (Participant, Care Provider, Investigator, Outcomes Assessor)|Primary Purpose: Treatment
- Enrollment - 110
- Age - 5 Years to 90 Years (Child, Adult, Older Adult)
- Outcome measures - Mean change from baseline score of Immune Response to end of the trial ( Time Frame: up to 4 weeks )|Mortality rate|Determine viral load in each patient
|
|
NCT04365582
|
OUTpatient Treatment of COVID-19 in Patients With Risk Factor for Poor Outcome |
Withdrawn |
Phase 3 |
May/07/2020 |
Apr/19/2021 |
- Alternative id - OUTCOV
- Interventions - Drug: Azithromycin|Drug: Hydroxychloroquine|Drug: Lopinavir 200Mg/Ritonavir 50Mg Tab
- Study type - Interventional
- Study results - No Results Available
- Locations -
- Study designs - Allocation: Randomized|Intervention Model: Parallel Assignment|Masking: None (Open Label)|Primary Purpose: Treatment
- Enrollment - 0
- Age - 50 Years and older (Adult, Older Adult)
- Outcome measures - Hospital admission|Effect of treatment on Death at D20|Effect of treatment on Death at D60|Effect of treatment on Death due to COVID at D20|Effect of treatment on Death due to COVID at D60|Effect of treatment on need for ICU stay at D20|Effect of treatment on need for ICU stay at D60|Effect of treatment on duration of ICU stay at D20|Effect of treatment on duration of ICU stay at D60|Effect of treatment on need of mechanical ventilation at D20|Effect of treatment on need of mechanical ventilation at D60|Effect of treatment on duration of mechanical ventilation at D20|Effect of treatment on duration of mechanical ventilation at D60|Effect of treatment on time to hospitalization at D20|Effect of treatment on time to hospitalization at D60|Effect of treatment on Duration of Hospital stay et D20|Effect of treatment on Duration of Hospital stay et D60|Effect of treatment on Duration of symptoms at D20|Effect of treatment on Duration of symptoms at D60|Incidence of Treatment-Emergent Adverse Events
|
|
NCT04345315
|
Correlative Study on Cancer Patients and Healthcare Professionals Exposed to Infection by Severe Acute Respiratory Syndrome-Corona Virus-2 (SARS-Cov-2), COVID-19 Causative Agent. |
Recruiting |
|
Mar/27/2020 |
Mar/01/2022 |
- Alternative id - IRSTB113
- Interventions - Other: serological test|Other: Rapid molecular test|Genetic: Next generation Sequencing (NGS) analysis|Other: serum chemistry analysis
- Study type - Observational
- Study results - No Results Available
- Locations - UO Microbiologia,Centro Servizi Pievesestina, AUSL Romagna, Cesena, FC, Italy|Irst Irccs, Meldola, FC, Italy
- Study designs - Observational Model: Cohort|Time Perspective: Other
- Enrollment - 500
- Age - 18 Years and older (Adult, Older Adult)
- Outcome measures - epidemiology|Immunoglobulin G (IgG) and Immunoglobulin M (IgM) antibodies evaluation|methods comparison|correlation between biochemical and coagulative factors with SARS-CoV-2 positivity.|phylogenetic map|interactions between the virus and host cells
|
|
NCT04331470
|
Evaluation of Efficacy of Levamisole and Formoterol+Budesonide in Treatment of COVID-19 |
Recruiting |
Phase 2|Phase 3 |
Apr/04/2020 |
May/20/2020 |
- Alternative id - 97548
- Interventions - Drug: Levamisole Pill + Budesonide+Formoterol inhaler|Drug: Lopinavir/Ritonavir + hydoxychloroquine
- Study type - Interventional
- Study results - No Results Available
- Locations - Vali-Asr Hospital, Fasa, Fars, Iran, Islamic Republic of
- Study designs - Allocation: Randomized|Intervention Model: Parallel Assignment|Masking: Double (Participant, Outcomes Assessor)|Primary Purpose: Treatment
- Enrollment - 30
- Age - 15 Years to 100 Years (Child, Adult, Older Adult)
- Outcome measures - Clear chest CT-scan|PCR test|Physical statues of patient
|
|
NCT04295551
|
Multicenter Clinical Study on the Efficacy and Safety of Xiyanping Injection in the Treatment of New Coronavirus Infection Pneumonia (General and Severe) |
Unknown status |
Not Applicable |
Mar/14/2020 |
Apr/14/2021 |
- Alternative id - QF-XYP1990-1
- Interventions - Drug: Lopinavir / ritonavir tablets combined with Xiyanping injection|Drug: Lopinavir/ritonavir treatment
- Study type - Interventional
- Study results - No Results Available
- Locations -
- Study designs - Allocation: Randomized|Intervention Model: Parallel Assignment|Masking: None (Open Label)|Primary Purpose: Treatment
- Enrollment - 80
- Age - 18 Years to 100 Years (Adult, Older Adult)
- Outcome measures - Clinical recovery time
|
|
NCT04350671
|
Interferon Beta 1a in Hospitalized COVID-19 Patients |
Enrolling by invitation |
Phase 4 |
Apr/15/2020 |
Apr/24/2020 |
- Alternative id - Interferon Beta 1a in COVID-19
- Interventions - Drug: Interferon Beta-1A|Drug: Lopinavir / Ritonavir|Drug: Single Dose of Hydroxychloroquine
- Study type - Interventional
- Study results - No Results Available
- Locations - Loghman Hakim Hospital, Shahid Beheshti University of Medical Sciences and Health Services, Tehran, Iran, Islamic Republic of
- Study designs - Allocation: Randomized|Intervention Model: Parallel Assignment|Masking: Triple (Participant, Care Provider, Investigator)|Primary Purpose: Treatment
- Enrollment - 40
- Age - 50 Years and older (Adult, Older Adult)
- Outcome measures - Time to clinical improvement|Mortality|SpO2 Improvement|Incidence of new mechanical ventilation use|Duration of hospitalization|Cumulative incidence of serious adverse events
|
|
NCT04779047
|
Comparative Therapeutic Efficacy and Safety of Different Antiviral and Anti Inflammatory Drugs in COVID-19 Patients. |
Recruiting |
Phase 4 |
Oct/01/2020 |
Apr/05/2021 |
- Alternative id - REC-H-PhBSU-21011
- Interventions - Drug: Remdesivir|Drug: Hydroxychloroquine|Drug: Tocilizumab|Drug: Lopinavir/ Ritonavir|Drug: Ivermectin
- Study type - Interventional
- Study results - No Results Available
- Locations - Beni-suef University, Banī Suwayf, Egypt
- Study designs - Allocation: Randomized|Intervention Model: Parallel Assignment|Masking: None (Open Label)|Primary Purpose: Treatment
- Enrollment - 150
- Age - 18 Years to 88 Years (Adult, Older Adult)
- Outcome measures - Percentage of clinical cure in each arm
|
|
NCT04568421
|
Argentinian Registry of Patients With Rheumatic Diseases and COVID-19 Infection |
Recruiting |
|
Aug/18/2020 |
Dec/31/2021 |
- Alternative id - 01
- Interventions - Drug: Immunosuppressive Agents
- Study type - Observational
- Study results - No Results Available
- Locations - Sociedad Argentina de Reumatología, Ciudad Autónoma de Buenos Aires, Caba, Argentina
- Study designs - Observational Model: Cohort|Time Perspective: Prospective
- Enrollment - 3000
- Age - 18 Years to 100 Years (Adult, Older Adult)
- Outcome measures - Mortality|Hospitalization|SARS-CoV-2 infection presentation|Admission at the intensive care unit|Invasive mechanical ventilation|COVID-19 Complications|Recovery rate
|
|
NCT04315948
|
Trial of Treatments for COVID-19 in Hospitalized Adults |
Recruiting |
Phase 3 |
Mar/22/2020 |
Mar/01/2023 |
- Alternative id - C20-15|101015736
- Interventions - Drug: Remdesivir|Drug: Lopinavir/ritonavir|Drug: Interferon Beta-1A|Drug: Hydroxychloroquine|Other: Standard of care|Drug: AZD7442|Other: Placebo
- Study type - Interventional
- Study results - No Results Available
- Locations - Medizinische Universität Innsbruck, Innsbruck, Austria|Kepler Universitätsklinikum Linz, Linz, Austria|Landeskrankenhaus Salzburg Universitätsklinikum der Paracelsus Medizinischen Privatuniversität, Salzburg, Austria|Hôpital Erasme - Cliniques universitaires de Bruxelles, Brussels, Belgium|Hôpital Saint Luc, Brussels, Belgium|CHU Brugmann, Brussels, Belgium|Hôpital La Citadelle, Liège, Belgium|Pôle Hospitalier Jolimont / site de Mons-Warquignies, Mons, Belgium|Fakultní nemocnice u sv. Anny v Brně, Brno, Czechia|Centre Hospitalier Universitaire Amiens-Picardie, Amiens, France|Centre Hospitalier Regional Metz-Thionville, Ars-Laquenexy, France|Centre Hospitalier Régional Universitaire de Besançon, Besançon, France|Centre Hospitalier Universitaire de Bordeaux, Bordeaux, France|CHU APHP Ambroise-Paré, Boulogne-Billancourt, France|Centre Hospitalier Andrée Rosemon, Cayenne, France|CHU Clermont-Ferrand, Clermont-Ferrand, France|Hospices Civil, Colmar, France|APHP - hôpital Henri-Mondor, Créteil, France|Centre Hospitalier Universitaire Dijon-Bourgogne, Dijon, France|Centre Hospitalier Universitaire de Martinique, Fort De France, France|AP-HP Hôpital Bicêtre, Kremlin-Bicêtre, France|Centre Hospitalo-Universitaire de Grenoble, La Tronche, France|Centre hospitalier de Versailles, Le Chesnay, France|Centre Hospitalier Régional Universitaire de Lille, Lille, France|Hospices Civils de Lyon, Lyon, France|Centre Hospitalier de Mont de Marsan, Mont-de-Marsan, France|Centre Hospitalier Universitaire de Montpellier, Montpellier, France|Groupe Hospitalier de la Région de Mulhouse Sud Alsace, Mulhouse, France|Centre Hospitalier Régional et Universitaire de Nancy, Nancy, France|Centre Hospitalier Universitaire de Nantes, Nantes, France|Centre Hospitalo-Universitaire de Nice, Nice, France|CHU Nîmes, Nîmes, France|APHP - Hôpital Lariboisière, Paris, France|APHP - Hôpital Saint Louis, Paris, France|APHP - Hôpital Saint Antoine, Paris, France|APHP - Hôpital Universitaire Pitié Salpêtrière, Paris, France|APHP - Hôpital Cochin, Paris, France|Hôpital Paris Saint-Joseph et Marie Lannelongue, Paris, France|APHP - Hôpital Necker, Paris, France|APHP- Hôpital Européen Georges-Pompidou, Paris, France|APHP - Hôpital Bichat Claude Bernard, Paris, France|APHP - Hôpital Tenon, Paris, France|CHU Poitiers, Poitiers, France|CH Cornouaille, Quimper, France|CHU de Reims, Reims, France|Centre Hospitalier Universitaire de Rennes, Rennes, France|Hopital DELAFONTAINE, Saint-Denis, France|Hôpital d'Instruction des Armées BEGIN, Saint-Mandé, France|Centre Hospitalier Universitaire de Saint Etienne, Saint-Étienne, France|Centre Hospitalier Régional Universitaire de Strasbourg, Strasbourg, France|Centre Hospitalier Universitaire de Toulouse, Toulouse, France|Centre Hospitalier Universitaire de Toulouse, Toulouse, France|Centre Hospitalier de Tourcoing, Tourcoing, France|Centre Hospitalier Universitaire de Tours, Tours, France|CH Bretagne Atlantique, Vannes, France|CH Bretagne Atlantique, Vannes, France|Centre Hospitalier Annecy Genevois, Épagny, France|Evaggelismos General Hospital, Athens, Greece|General University Hospital of Patras, Patras, Greece|hospital Saint James, Dublin, Ireland|Centre Hospitalier Luxembourg, Luxembourg, Luxembourg|Hôpitaux Robert Schuman, Luxembourg, Luxembourg|Akershus Unniversity Hospital, Oslo, Norway|Lovisenberg Diaconal Hospital, Oslo, Norway|Oslo University Hospital, Oslo, Norway|Bienganski Hospital, Łódź, Poland|Hospital de Abrantes (CHMT), Abrantes, Portugal|Hospital de Cascais, Cascais, Portugal|CHULN- Hospital de Santa Maria, Lisboa, Portugal|CHULN- Hospital de Santa Maria, Lisboa, Portugal|Centro Hospitalar Universitário de São João, EPE, Porto, Portugal|Martin University Hospital, Martin, Slovakia
- Study designs - Allocation: Randomized|Intervention Model: Parallel Assignment|Masking: Triple (Participant, Investigator, Outcomes Assessor)|Primary Purpose: Treatment
- Enrollment - 2416
- Age - 18 Years and older (Adult, Older Adult)
- Outcome measures - Percentage of subjects reporting each severity rating on a 7-point ordinal scale|Status on an ordinal scale|National Early Warning Score 2 (NEWS-2 score)|Number of oxygenation free days in the first 28 days|Incidence of new oxygen use, non-invasive ventilation or high flow oxygen devices during the trial.|Ventilator free days in the first 28 days|Incidence of new mechanical ventilation use during the trial.|Need for mechanical ventilation or death by Day 15|Hospitalization|Mortality|Occurrence of new hospitalization|Occurrence of confirmed re-infection with SARS-CoV-2|Cumulative incidence of serious adverse events (SAEs)|Cumulative incidence of Grade 1- 2 hypersensitivity- related and infusion related AEs until D29 visit|Cumulative incidence of Grade 3 and 4 adverse events (AEs)|Number of participants with a discontinuation or temporary suspension of study drugs (for any reason)|Cumulative incidence of AEs of Special Interest
|
|
NCT04409483
|
Evaluation of Additional Treatments for COVID-19: a Randomized Trial in Niger |
Withdrawn |
Phase 3 |
Jun/01/2020 |
Dec/31/2020 |
- Alternative id - Trascov
- Interventions - Drug: Lopinavir-Ritonavir Drug Combination|Combination Product: Standard Care
- Study type - Interventional
- Study results - No Results Available
- Locations -
- Study designs - Allocation: Randomized|Intervention Model: Parallel Assignment|Masking: None (Open Label)|Primary Purpose: Treatment
- Enrollment - 0
- Age - 12 Years and older (Child, Adult, Older Adult)
- Outcome measures - Hospitalization or death|All-cause mortality|Time to hospitalization|Length of hospitalization|Admission to intensive care|Adverse events|Serious adverse events
|
|
NCT04328285
|
Chemoprophylaxis of SARS-CoV-2 Infection (COVID-19) in Exposed Healthcare Workers |
Active, not recruiting |
Phase 3 |
Apr/14/2020 |
Mar/30/2022 |
- Alternative id - 20PH061|2020-001188-96
- Interventions - Drug: Hydroxychloroquine|Drug: Placebo of Hydroxychloroquine|Drug: Lopinavir and ritonavir|Drug: Placebo of LPV/r Tablets
- Study type - Interventional
- Study results - No Results Available
- Locations - CHU d'Angers, Angers, France|CHU de Bordeaux, Bordeaux, France|CHU de Clermont-Ferrand, Clermont-ferrand, France|CHU de Montpellier, Montpellier, France|CHU de Nancy, Nancy, France|CHU de Nantes, Nantes, France|CHU de Rennes, Rennes, France|CHU de Rouen, Rouen, France|CHU de Saint-Etienne, Saint-Étienne, France
- Study designs - Allocation: Randomized|Intervention Model: Parallel Assignment|Masking: Triple (Participant, Investigator, Outcomes Assessor)|Primary Purpose: Prevention
- Enrollment - 1200
- Age - 18 Years and older (Adult, Older Adult)
- Outcome measures - Occurrence of an symptomatic or asymptomatic SARS-CoV-2 infection among healthcare workers (HCWs)|Evaluation of the occurrence of adverse events in each arm,|Evaluation of the discontinuation rates of the investigational drug in each arm,|Evaluation of the adherence of participants to study drug,|Evaluation of the incidence of symptomatic cases of SARS-CoV-2 infection in each arm,|Evaluation of the incidence of asymptomatic cases of SARS-CoV-2 infection in each arm|Evaluation of the incidence of severe cases of SARS-CoV-2 infection in each arm.|corrected QT interval (ms)
|
|
NCT04425382
|
Darunavir/Cobicistat vs. Lopinavir/Ritonavir in COVID-19 Pneumonia in Qatar |
Recruiting |
|
Mar/01/2020 |
Sep/01/2020 |
- Alternative id - MRC-05-069
- Interventions - Drug: Darunavir/Cobicistat|Drug: Lopinavir/Ritonavir
- Study type - Observational
- Study results - No Results Available
- Locations - Hamad Medical Corporation, Doha, Qatar
- Study designs - Observational Model: Other|Time Perspective: Retrospective
- Enrollment - 200
- Age - 18 Years and older (Adult, Older Adult)
- Outcome measures - Time to Clinical Improvement and/or Virological Clearance (Composite Endpoint)|Percentage of Virological Clearance|Percentage of Clinical Deterioration|Incidence of Adverse Events|Length of Hospital Stay|All-cause Mortality
|
|
NCT04276688
|
Lopinavir/ Ritonavir, Ribavirin and IFN-beta Combination for nCoV Treatment |
Completed |
Phase 2 |
Feb/10/2020 |
Mar/31/2020 |
- Alternative id - UW-20-074
- Interventions - Drug: Lopinavir/ritonavir|Drug: Ribavirin|Drug: Interferon Beta-1B
- Study type - Interventional
- Study results - No Results Available
- Locations - University of Hong Kong, Queen Mary Hospital, Hong Kong, Hong Kong
- Study designs - Allocation: Randomized|Intervention Model: Parallel Assignment|Masking: None (Open Label)|Primary Purpose: Treatment
- Enrollment - 127
- Age - 18 Years and older (Adult, Older Adult)
- Outcome measures - Time to negative NPS|Time to negative saliva|Time to clinical improvement|Hospitalisation|Mortality|Immune reaction|Adverse events|Time to negative all clinical specimens
|
|
NCT04542408
|
Hamburg Edoxaban for Anticoagulation in COVID-19 Study |
Recruiting |
Phase 3 |
Nov/12/2020 |
Sep/01/2022 |
- Alternative id - HERO-19
- Interventions - Drug: Anticoagulation Agents (Edoxaban and/or high dose LMWH)|Drug: Low dose Low molecular weight heparin or Placebo
- Study type - Interventional
- Study results - No Results Available
- Locations - UK Aachen, Aachen, Germany|Universitätsklinikum Augsburg, Augsburg, Germany|Universitätsklinikum Düsseldorf, Düsseldorf, Germany|Universitätsklinikum Freiburg, Freiburg, Germany|Asklepios Klinik Altona, Hamburg, Germany|Asklepios Klinik Barmbek, Hamburg, Germany|Asklepios Klinik St. Georg, Hamburg, Germany|Universitärsklinikum Hamburg-Eppendorf, Hamburg, Germany|Medizinische Hochschule Hannover, Hanover, Germany|TU München Klinikum rechts der Isar, München, Germany
- Study designs - Allocation: Randomized|Intervention Model: Parallel Assignment|Masking: Double (Investigator, Outcomes Assessor)|Primary Purpose: Treatment
- Enrollment - 172
- Age - 18 Years and older (Adult, Older Adult)
- Outcome measures - Combined endpoint: all-cause mortality and/ or venous thromboem-bolism and/ or arterial thromboembolism|All-cause mortality|Mortality related to venous thromboembolism|Mortality related to arterial thromboembolism|Rate of venous and/ or arterial thromboembolism|Rate and length of mechanical ventilation|Length of initial stay at ICU after application of IMP|Rehospitalisation|Rate and length of renal replacement therapy|Cardiac arrest/ CPR
|
|
NCT03376854
|
Pilot RCT of Therapeutic Hypothermia Plus Neuromuscular Blockade in COVID-19 Patients With ARDS |
Withdrawn |
Phase 2 |
May/01/2018 |
Apr/27/2021 |
- Alternative id - HP-00078506
- Interventions - Device: Hypothermia|Drug: Neuromuscular Blocking Agents|Device: Standard of Care
- Study type - Interventional
- Study results - No Results Available
- Locations - University of Maryland Medical Center, Baltimore, Maryland, United States
- Study designs - Allocation: Randomized|Intervention Model: Parallel Assignment|Masking: None (Open Label)|Primary Purpose: Treatment
- Enrollment - 0
- Age - 18 Years to 65 Years (Adult, Older Adult)
- Outcome measures - Targeted temperature compliance|Adverse event|28-day ICU-free days|Survival|non neurologic Sequential Organ Failure (SOFA) scores|Oxygen saturation (SpO2)|Plateau airway pressure|Mean airway pressure|Airway driving pressure|Oxygen saturation index|Core temperature|Urine output|comprehensive metabolic panel|Complete blood count with differential count and platelet count|Biomarkers|Serum electrolytes|Blood glucose|28-day ventilator-free days
|
|
NCT04559074
|
Personalised Electronic Record Supported Optimisation When Alone for Patients With Hypertension- Pilot Study for Remote Medical Management of Hypertension During the COVID-19 Pandemic |
Recruiting |
Phase 4 |
Oct/23/2020 |
Jul/31/2021 |
- Alternative id - 283209
- Interventions - Drug: Amlodipine
- Study type - Interventional
- Study results - No Results Available
- Locations - Queen Mary University London, London, United Kingdom
- Study designs - Allocation: Non-Randomized|Intervention Model: Parallel Assignment|Masking: None (Open Label)|Primary Purpose: Treatment
- Enrollment - 1000
- Age - 18 Years and older (Adult, Older Adult)
- Outcome measures - Reductions in blood pressure in participants with primary hypertension and inadequate BP control by up-titration of amlodipine in 1-2 mg increments.|Mean change in daily DBP|Difference between mean changes of blood pressure|Collect data on tolerability / side effects
|
|
NCT04275388
|
Xiyanping Injection for the Treatment of New Coronavirus Infected Pneumonia |
Not yet recruiting |
|
May/15/2020 |
Dec/14/2021 |
- Alternative id - QF-XYP2001-1
- Interventions - Drug: Xiyanping injection|Drug: Lopinavir / ritonavir, alpha-interferon nebulization,Abidor Hydrochloride
- Study type - Observational
- Study results - No Results Available
- Locations -
- Study designs - Observational Model: Cohort|Time Perspective: Retrospective
- Enrollment - 426
- Age - up to 100 Years (Child, Adult, Older Adult)
- Outcome measures - Clinical recovery time|Complete fever time|Cough relief time|Virus negative time|Incidence of severe or critical neocoronavirus pneumonia
|
|
NCT04590586
|
Study of Multiple Candidate Agents for the Treatment of COVID-19 in Hospitalized Patients |
Completed |
Phase 3 |
Nov/24/2020 |
Aug/03/2021 |
- Alternative id - COV-01
- Interventions - Drug: Standard of care|Drug: Apremilast|Drug: Apremilast placebo|Drug: Lanadelumab|Drug: Lanadelumab placebo|Drug: Zilucoplan|Drug: Zilucoplan placebo
- Study type - Interventional
- Study results - No Results Available
- Locations - Pinnacle Research Group LLC, Anniston, Alabama, United States|Good Samaritan Hospital, Bakersfield, California, United States|Sharp Chula Vista Medical Center, Chula Vista, California, United States|El Centro Regional Medical Center, El Centro, California, United States|University of California Irvine Medical Center, Orange, California, United States|Riverside Community Hospital, Riverside, California, United States|National Institute of Clinical Research, S. El Monte, California, United States|University of California Davis Health System, Sacramento, California, United States|UF Health Shands Hospital, Gainesville, Florida, United States|Memorial Hospital Jacksonville, Jacksonville, Florida, United States|Grady Health System, Atlanta, Georgia, United States|Great Lakes Clinical Trials, Chicago, Illinois, United States|The University of Iowa, Iowa City, Iowa, United States|Harper University Hospital, Detroit, Michigan, United States|Sinai Grace Hospital, Detroit, Michigan, United States|Detroit Receiving Hospital, Royal Oak, Michigan, United States|University of Tennessee Health Sciences Center, Memphis, Tennessee, United States|Medical City Ft. Worth, Fort Worth, Texas, United States|University of Texas Health Science Center at Houston, Houston, Texas, United States|Texoma Medical Center, Sherman, Texas, United States|MultiCare Health System Institute for Research and Innovation, Tacoma, Washington, United States|Hospital San Juan de Dios, Ramos Mejia, Buenos Aires, Argentina|Hospital General de Agudos Dr. J. M. Ramos Mejia, Ciudad Autonoma Buenos Aires, Argentina|Hospital General de Agudos Dr. Ignacio Pirovano, Ciudad Autonoma Buenos Aires, Argentina|Clinica Adventista Belgrano, Ciudad Autonoma Buenos Aires, Argentina|Hospital Italiano de Rosario, Rosario, Argentina|Chronos Pesquisa Clinica, Brasília, Distrito Federal, Brazil|HC-UFG - Hospital das Clínicas da Universidade Federal de Goiás, Goiânia, Goiás, Brazil|Santa Casa de Misericórdia de Belo Horizonte, Belo Horizonte, Minas Gerais, Brazil|Hospital de Clínicas de Porto Alegre, Porto Alegre, Rio Grande Do Sul, Brazil|UNESP - Faculdade de Medicina da Universidade Estadual Paulista - Campus Botucatu, Botucatu, Sao Paulo, Brazil|Praxis Pesquisa Médica, Santo André, Sao Paulo, Brazil|Hospital Base Osorno, Osorno, Chile|Hospital General de Tijuana, Tijuana, Baja California Norte, Mexico|Hospital Civil de Guadalajara Dr. Juan I. Menchaca, Guadalajara, Jalisco, Mexico|Universidad Autonoma de Nuevo Leon, Hospital Universitario Dr. Jose Eleuterio Gonzalez, Monterrey, Nuevo León, Mexico|Hospital Civil de Culiacan, Culiacán, Sinaloa, Mexico|TSBIH "Krasnoyarsk Interdistrict Clinical Hospital of Emergency Medical Care n.a. N.S. Karpovich, Krasnoyarsk, Russian Federation|SBIH of Moscow "Infectious Clinical Hospital # 1 of Department of Healthcare of Moscow", Moscow, Russian Federation|SPb SBIH "Alexandrovskaya City Hospital", Saint Petersburg, Russian Federation|St-George Hospital, Saint Petersburg, Russian Federation|SPb SBIH "Nikolaevskaya Hospital", Saint Petersburg, Russian Federation|SPb SBIH "City Pokrovskaya Hospital", Saint Petersburg, Russian Federation|SPb SBIH "City Hospital # 40 of Kurortnyi region", Sestroretsk, Russian Federation|Nelson Mandela Academic Clinical Research Unit (NeMACRU), Mthatha, Eastern Cape, South Africa|Johese Clinical Research: Unitas, Centurion, Gauteng, South Africa|MERC SiReN, Johannesburg, Gauteng, South Africa|Drs Sarvan and Moodley, Durban, KwaZulu-Natal, South Africa|Tread Research, Cape Town, Western Cape, South Africa|Tiervlei Trial Centre, Cape Town, Western Cape, South Africa|2 Military Hospital Internal Medicine, Cape Town, Western Cape, South Africa|Dr JM Engelbrecht Trial Site, Somerset West, Western Cape, South Africa|Clinical Projects Research SA (PTY) LTD, Worcester, Western Cape, South Africa|Communal Noncommercial Profit "Clinical City Hospital 16 of Dnipro Regional Council", Dnipro, Ukraine|CNE of Kharkov RC Reg Cl Infectious Hospital, Kharkiv, Ukraine|Communal Non-Commercial Medical Enterprise "O.T.Bohayevskyi Kremenchuk City Hospital #1", Kremenchuk, Ukraine|City Clinical infectious Hospital, Odesa, Ukraine|Municipal Non-Profit Enterprise Central City Hospital Of Rivne City Council, Rivne, Ukraine|CCH #1 Vinnytsia M.I.Pyrogov NMU Ch of Infectious Diseases, Vinnytsia, Ukraine
- Study designs - Allocation: Randomized|Intervention Model: Parallel Assignment|Masking: Quadruple (Participant, Care Provider, Investigator, Outcomes Assessor)|Primary Purpose: Treatment
- Enrollment - 516
- Age - 18 Years and older (Adult, Older Adult)
- Outcome measures - Time to confirmed clinical recovery|Number of participants who achieve oxygen-free recovery at Day 29|Number of participants who experience ≥2-point improvement from baseline or assessed as fit-for-discharge on the ordinal scale at Day 29|Incidence of all-cause mortality|Distribution of participants across the 8-point ordinal scale of clinical severity status scores at Day 8, Day 15 and Day 29|Worst post-baseline score on the 8-point ordinal scale of clinical severity status|Number of intensive care unit (ICU) days|Number of invasive mechanical ventilator days|Number of participants who achieve clinical recovery at Day 8, Day 15, and Day 29|Number of participants who achieve sustained clinical recovery at Day 60|Number of participants who experience one or more treatment-emergent adverse events (TEAEs)|Number of participants who experience one or more serious adverse events (SAEs)|Number of participants who experience one or more common terminology criteria for adverse events (CTCAE) grade 3 or higher|Number of participants who experience one or more adverse events (AEs) leading to dose modification|Number of participants who experience one or more adverse events (AEs) leading to discontinuation
|
|
NCT04381936
|
Randomised Evaluation of COVID-19 Therapy |
Recruiting |
Phase 2|Phase 3 |
Mar/19/2020 |
Nov/01/2032 |
- Alternative id - NDPHRECOVERY|2020-001113-21|ISRCTN50189673
- Interventions - Drug: Lopinavir-Ritonavir|Drug: Corticosteroid|Drug: Hydroxychloroquine|Drug: Azithromycin|Biological: Convalescent plasma|Drug: Tocilizumab|Biological: Immunoglobulin|Drug: Synthetic neutralising antibodies|Drug: Aspirin|Drug: Colchicine|Drug: Baricitinib|Drug: Anakinra|Drug: Dimethyl fumarate|Drug: High Dose Corticosteroid|Drug: Empagliflozin|Drug: Sotrovimab
- Study type - Interventional
- Study results - No Results Available
- Locations - Kumasi Center for Collaborative Research in Tropical Medicine KNUST, Kumasi, Ghana|Indian Council of Medical Research, Division of Epidemiology and Communicable Diseases, New Delhi, India|Eijkman Oxford Clinical Research Unit (EOCRU), Eijkman Institute for Molecular Biology, Jakarta, Indonesia|Clinical Trial Unit, Oxford University Clinical Research Unit-Nepal, Patan Academy of Health Sciences, Kathmandu, Nepal|Wits Health Consortium, Johannesburg, South Africa|RECOVERY Sri Lanka & Pakistan, National Intensive Care Surveillance - M.O.R.U, Colombo, Sri Lanka|Nuffield Department of Population Health, University of Oxford, Oxford, United Kingdom|Oxford University Clinical Research Unit, Centre for Tropical Medicine, Ho Chi Minh City, Vietnam
- Study designs - Allocation: Randomized|Intervention Model: Factorial Assignment|Masking: None (Open Label)|Primary Purpose: Treatment
- Enrollment - 50000
- Age - Child, Adult, Older Adult
- Outcome measures - All-cause mortality|Duration of hospital stay|Composite endpoint of death or need for mechanical ventilation or ECMO
|
|
NCT04386876
|
Bioequivalence Study of Lopinavir/Ritonavir 200/50 mg Film Tablet (World Medicine Ilac, Turkey) Under Fasting Conditions |
Completed |
Phase 1 |
Apr/30/2020 |
Jun/11/2020 |
- Alternative id - NOV2020/01911|FARGE365
- Interventions - Drug: Lopinavir/Ritonavir 200 mg/50 mg Film Tablet|Drug: Lopinavir/Ritonavir 200 mg/50 mg Film Coated Tablet
- Study type - Interventional
- Study results - No Results Available
- Locations - Novagenix Drug R&D Center, Akyurt, Ankara, Turkey|Farmagen Ar-Ge Biyot. Ltd. Sti., Sahinbey, Gaziantep, Turkey
- Study designs - Allocation: Randomized|Intervention Model: Crossover Assignment|Masking: None (Open Label)|Primary Purpose: Other
- Enrollment - 30
- Age - 20 Years to 40 Years (Adult)
- Outcome measures - Primary PK End Points
|
|
NCT04468087
|
Antiviral Agents Against COVID-19 Infection |
Active, not recruiting |
Phase 2|Phase 3 |
Feb/15/2021 |
Dec/31/2022 |
- Alternative id - REVOLUTIOn
- Interventions - Drug: Atazanavir|Drug: Daclatasvir 60 mg|Drug: Sofusbuvir + Daclastavir 60 mg|Drug: Placebo Atazanavir|Drug: Placebo Daclatasvir 60 mg|Drug: Placebo Sofusbuvir + Daclatasvir 60 mg
- Study type - Interventional
- Study results - No Results Available
- Locations - Hospital do Coracao, Sao Paulo, SP, Brazil
- Study designs - Allocation: Randomized|Intervention Model: Parallel Assignment|Masking: Quadruple (Participant, Care Provider, Investigator, Outcomes Assessor)|Primary Purpose: Treatment
- Enrollment - 256
- Age - 18 Years to 90 Years (Adult, Older Adult)
- Outcome measures - Phase II first step: Change in the slope of SARS-COV 2 viral load|Phase II second step: Change in the slope of SARS-COV 2 viral load|Phase III: Number of free days from respiratory support
|
|
NCT04261907
|
Evaluating and Comparing the Safety and Efficiency of ASC09/Ritonavir and Lopinavir/Ritonavir for Novel Coronavirus Infection |
Unknown status |
Not Applicable |
Feb/07/2020 |
Jun/30/2020 |
- Alternative id - ASC09F-CTP-ZY-01
- Interventions - Drug: ASC09/ritonavir group|Drug: lopinavir/ritonavir group
- Study type - Interventional
- Study results - No Results Available
- Locations -
- Study designs - Allocation: Randomized|Intervention Model: Parallel Assignment|Masking: None (Open Label)|Primary Purpose: Treatment
- Enrollment - 160
- Age - 18 Years to 75 Years (Adult, Older Adult)
- Outcome measures - The incidence of composite adverse outcome|Time to recovery|Rate of no fever|Rate of no cough|Rate of no dyspnea|Rate of no requring supplemental oxygen|Rate of undectable viral RNA|Rate of mechanical ventilation|Rate of ICU admission|Time and rate of laboratory indicators related to disease improvement to return to normal
|
|
NCT04455958
|
Lopinavir/Ritonavir for the Treatment of COVID-19 Positive Patients With Cancer and Immune Suppression in the Last Year |
Withdrawn |
Phase 2 |
May/01/2021 |
Nov/01/2021 |
- Alternative id - STUDY00021444|NCI-2020-02877
- Interventions - Drug: Lopinavir/Ritonavir|Drug: Placebo Administration|Other: Questionnaire Administration
- Study type - Interventional
- Study results - No Results Available
- Locations -
- Study designs - Allocation: Randomized|Intervention Model: Parallel Assignment|Masking: Double (Participant, Investigator)|Primary Purpose: Treatment
- Enrollment - 0
- Age - 18 Years and older (Adult, Older Adult)
- Outcome measures - Severity of symptoms|Clinical benefit rate of lopinavir/ritonavir|Time to symptom progression|Time to improvement of participants|Time to hospital admission for those who develop severe of critical symptoms|Intensive care unit (ICU) admission: yes or no|Receiving ventilator support: yes or no|Overall survival
|
|
NCT05195060
|
TURN-COVID Biobank: The Dutch Cohort Study for the Evaluation of the Use of Neutralizing Monoclonal Antibodies and Other Antiviral Agents Against SARS-CoV-2 |
Recruiting |
|
Dec/14/2021 |
Jun/14/2024 |
- Alternative id - NL78705.018.21
- Interventions - Drug: casirivimab with imdevimab|Drug: sotrovimab|Drug: molnupiravir
- Study type - Observational
- Study results - No Results Available
- Locations - Amsterdam University Medical centre - VUMC, Amsterdam, Noord Holland, Netherlands|Amsterdam University Medical Centre, Amsterdam, Noord-Holland, Netherlands|Leiden universitair medisch centrum, Leiden, Netherlands|Radboud Universitair Medisch Centrum, Nijmegen, Netherlands
- Study designs - Observational Model: Cohort|Time Perspective: Prospective
- Enrollment - 1000
- Age - 18 Years and older (Adult, Older Adult)
- Outcome measures - Therapeutic effect of treatment with monoclonal antibodies and antiviral agents|Incidence of Treatment-Emergent Adverse Events of treatment with monoclonal antibodies and antiviral agents|Cost-effectiveness of treatment with monoclonal antibodies and antiviral agents|Change of serologic response during treatment with monoclonal antibodies and antiviral agents
|
|
NCT04701710
|
Prophylaxis Covid-19 in Healthcare Agents by Intensive Treatment With Ivermectin and Iota-carrageenan |
Completed |
Phase 1|Phase 2 |
Oct/15/2020 |
Dec/31/2020 |
- Alternative id - 5076-410-CH2020
- Interventions - Drug: Ivermectin / Iota-Carrageenan
- Study type - Interventional
- Study results - No Results Available
- Locations - SI.PRO.SA, Ministerio de Salud Pública, Tucumán, Argentina
- Study designs - Allocation: Randomized|Intervention Model: Parallel Assignment|Masking: None (Open Label)|Primary Purpose: Prevention
- Enrollment - 300
- Age - 18 Years to 60 Years (Adult)
- Outcome measures - Pearson's Chi-square and proportion test.|Odd Ratio, probabilistic test|Logistic regression test
|
|
NCT04354428
|
Treatment for COVID-19 in High-Risk Adult Outpatients |
Active, not recruiting |
Phase 2|Phase 3 |
Apr/16/2020 |
Jan/01/2021 |
- Alternative id - STUDY00009878|INV-017062
- Interventions - Drug: Ascorbic Acid|Drug: Hydroxychloroquine Sulfate|Drug: Azithromycin|Drug: Folic Acid|Drug: Lopinavir 200 MG / Ritonavir 50 MG [Kaletra]
- Study type - Interventional
- Study results - No Results Available
- Locations - Ruth M. Rothstein CORE Center - Cook County Health, Chicago, Illinois, United States|Tulane University, New Orleans, Louisiana, United States|Boston University, Boston, Massachusetts, United States|SUNY Upstate Medical University, Syracuse, New York, United States|University of Washington Coordinating Center, Seattle, Washington, United States|UW Virology Research Clinic, Seattle, Washington, United States
- Study designs - Allocation: Randomized|Intervention Model: Parallel Assignment|Masking: Double (Participant, Investigator)|Primary Purpose: Treatment
- Enrollment - 300
- Age - 18 Years to 80 Years (Adult, Older Adult)
- Outcome measures - Lower respiratory tract infection (LRTI) rates|Incidence of hospitalization or mortality|Change in upper respiratory viral shedding|COVID-19 symptom resolution rates [Lopinavir-ritonavir arm only]|Rate of participant-reported adverse events|COVID-19-related hospitalization days|Rate of disease severity|Viral shedding rates|Individual lopinavir-ritonavir concentration profiles and exposure estimates [Lopinavir-ritonavir arm only]
|
|
NCT04343768
|
An Investigation Into Beneficial Effects of Interferon Beta 1a, Compared to Interferon Beta 1b And The Base Therapeutic Regiment in Moderate to Severe COVID-19: A Randomized Clinical Trial |
Completed |
Phase 2 |
Apr/09/2020 |
Apr/27/2020 |
- Alternative id - Different Interferons in COVID
- Interventions - Drug: Hydroxychloroquine|Drug: Lopinavir / Ritonavir|Drug: Interferon Beta-1A|Drug: Interferon Beta-1B
- Study type - Interventional
- Study results - No Results Available
- Locations - Loghman Hakim Hospital, Shahid Beheshti University of Medical Sciences and Health Services, Tehran, Iran, Islamic Republic of
- Study designs - Allocation: Randomized|Intervention Model: Parallel Assignment|Masking: None (Open Label)|Primary Purpose: Treatment
- Enrollment - 60
- Age - 18 Years and older (Adult, Older Adult)
- Outcome measures - Time to clinical improvement|Mortality|SpO2 Improvement|Incidence of new mechanical ventilation use|Duration of hospitalization
|
|
NCT04372628
|
Trial of Early Therapies During Non-hospitalized Outpatient Window for COVID-19 |
Active, not recruiting |
Phase 2 |
Jun/01/2020 |
Jun/01/2022 |
- Alternative id - 200827
- Interventions - Drug: Lopinavir/Ritonavir 400 mg/100 mg|Other: Placebo
- Study type - Interventional
- Study results - No Results Available
- Locations - University of Colorado School of Medicine, Aurora, Colorado, United States|Beth Israel Deaconess Medical Center, Boston, Massachusetts, United States|University of Mississippi Medical Center, Jackson, Mississippi, United States|Vanderbilt University Medical Center, Nashville, Tennessee, United States|Intermountain, Murray, Utah, United States|University of Wisconsin, Madison, Wisconsin, United States
- Study designs - Allocation: Randomized|Intervention Model: Sequential Assignment|Masking: Triple (Participant, Care Provider, Investigator)|Primary Purpose: Treatment
- Enrollment - 600
- Age - 18 Years and older (Adult, Older Adult)
- Outcome measures - Modified COVID Ordinal Outcomes Scale: Study Day 15|Modified COVID Ordinal Outcome Scale: Study Day 8|Modified COVID Ordinal Outcome Scale: Study Day 29|Proportion of patients hospitalized: Day 1 to 29|Time to hospitalization Day 1 to Day 29|Time to symptom resolution: Day 1 to Day 29|All-cause, all-location mortality: Day 1 to Day 29|Oxygen-free days: Day 1 to Day 29|Fever-free days: Day 1 to Day 29|Ventilator-free days: Day 1 to Day 29|ICU-free days: Day 1 to Day 29|Hospital-free days: Day 1 to Day 29
|
|
NCT04364022
|
Efficacy of Pragmatic Same-day COVID-19 Ring Prophylaxis for Adult Individuals Exposed to SARS-CoV-2 in Switzerland |
Completed |
Phase 3 |
Apr/23/2020 |
Mar/24/2021 |
- Alternative id - CCER 2020-00864
- Interventions - Drug: Lopinavir/ritonavir
- Study type - Interventional
- Study results - No Results Available
- Locations - Instituto Nacional de Infectiologia Evandro Chagas, Fiocruz, Rio De Janeiro, Brazil|Universitätsspital Basel and SwissTPH, Basel, Switzerland|Hôpitaux Universitaires de Genève, Geneva, Switzerland|Ospedale Regionale di Lugano, Lugano, Switzerland
- Study designs - Allocation: Randomized|Intervention Model: Parallel Assignment|Masking: None (Open Label)|Primary Purpose: Prevention
- Enrollment - 326
- Age - 16 Years and older (Child, Adult, Older Adult)
- Outcome measures - 21-day incidence of COVID-19 in individuals exposed to SARS-CoV- 2 who are asymptomatic at baseline (intent-to-treat (ITT) analysis).|21-day incidence of COVID-19 in individuals exposed to SARS-CoV- 2 who are asymptomatic, PCR-confirmed SARS-CoV-2 negative and have negative SARS-CoV-2 serology at baseline (modified ITT)|21-day incidence of SARS-CoV-2 infection in individuals exposed to SARS-CoV-2 who are asymptomatic, PCR-confirmed SARS-CoV-2 negative and have negative SARS-CoV-2 serology at baseline (modified ITT)|Severity of clinical COVID-19 on a 7-point ordinal scale
|
|
NCT04252885
|
The Efficacy of Lopinavir Plus Ritonavir and Arbidol Against Novel Coronavirus Infection |
Completed |
Phase 4 |
Jan/28/2020 |
May/31/2020 |
- Alternative id - GZ8H-V1.0 20200122
- Interventions - Drug: Lopinavir and Ritonavir Tablets|Drug: Arbidol
- Study type - Interventional
- Study results - No Results Available
- Locations - Guangzhou Eighth People's Hospital, Guangzhou, Guangdong, China
- Study designs - Allocation: Randomized|Intervention Model: Parallel Assignment|Masking: None (Open Label)|Primary Purpose: Treatment
- Enrollment - 86
- Age - 18 Years to 80 Years (Adult, Older Adult)
- Outcome measures - The rate of virus inhibition|The disease prorogation-temperature|The disease prorogation-respiratory function 1|The disease prorogation-respiratory function 2|The disease prorogation-respiratory function 3
|
|
NCT04286503
|
The Clinical Study of Carrimycin on Treatment Patients With COVID-19 |
Unknown status |
Phase 4 |
Feb/23/2020 |
Feb/28/2021 |
- Alternative id - BeijingYouan Hospital
- Interventions - Drug: Carrimycin|Drug: lopinavir/ritonavir tablets or Arbidol or chloroquine phosphate|Drug: basic treatment
- Study type - Interventional
- Study results - No Results Available
- Locations -
- Study designs - Allocation: Randomized|Intervention Model: Parallel Assignment|Masking: None (Open Label)|Primary Purpose: Treatment
- Enrollment - 520
- Age - 18 Years to 75 Years (Adult, Older Adult)
- Outcome measures - Fever to normal time (day)|Pulmonary inflammation resolution time (HRCT) (day)|Negative conversion (%) of 2019-nCOVRNA in gargle (throat swabs) at the end of treatment
|
|
NCT04568096
|
Combination of Chemopreventive Agents (All- Trans Retinoic Acid and Tamoxifen) as Potential Treatment for the Lung Complication of COVID-19 |
Not yet recruiting |
Phase 2 |
Nov/01/2020 |
Dec/01/2020 |
- Alternative id - COVID-2019
- Interventions - Combination Product: Aerosolized All-Trans Retinoic acid plus oral Tamoxifen|Other: Standard treatment
- Study type - Interventional
- Study results - No Results Available
- Locations -
- Study designs - Allocation: Randomized|Intervention Model: Parallel Assignment|Masking: None (Open Label)|Primary Purpose: Treatment
- Enrollment - 160
- Age - 18 Years to 80 Years (Adult, Older Adult)
- Outcome measures - lung injury score|Angiotensin 1-7 (Ang 1-7) changes over time|Angiotensin 1-5 (Ang 1-5) changes over time|Renin changes over time|Aldosterone changes over time|Angiotensin-converting enzyme (ACE) changes over time|Frequency of adverse events and severe adverse events|Angiotensin II (Ang II) changes over time|Sequential organ failure assessment score(SOFA score) over time|Transe membrane protease ,serine II (TMPRSS2) changes over time|Testosterone levels changes over time|Dihydrotestosterone(DHT) levels changes over time|Thrombin time (TT)
|
|
NCT04922814
|
Comparison for the Effect of Neuromuscular Blocking Agents Versus Sedation Alone on Severe ARDS Patients Due to COVID-19 |
Not yet recruiting |
Not Applicable |
Aug/01/2021 |
Aug/01/2022 |
- Alternative id - Covid-19 ICU
- Interventions - Drug: muscle relaxation
- Study type - Interventional
- Study results - No Results Available
- Locations -
- Study designs - Allocation: Randomized|Intervention Model: Parallel Assignment|Masking: None (Open Label)|Primary Purpose: Supportive Care
- Enrollment - 40
- Age - 18 Years to 75 Years (Adult, Older Adult)
- Outcome measures - PaO2/FiO2|Change in lung mechanics|SOFA score|Measurement of tissue perfusion|Monitoring of Alveolar - Arterial Oxygen difference|28 days survival|Recording risk factors|Recording complications
|
|
NCT04374019
|
Novel Agents for Treatment of High-risk COVID-19 Positive Patients |
Terminated |
Phase 2 |
May/01/2020 |
Jan/12/2022 |
- Alternative id - MCC-20-COVID-01-PMC
- Interventions - Drug: Ivermectin|Drug: Camostat Mesilate|Dietary Supplement: Artemesia annua|Drug: Artesunate
- Study type - Interventional
- Study results - Has Results
- Locations - University of Kentucky Markey Cancer Center, Lexington, Kentucky, United States
- Study designs - Allocation: Randomized|Intervention Model: Parallel Assignment|Masking: None (Open Label)|Primary Purpose: Treatment
- Enrollment - 13
- Age - 18 Years to 99 Years (Adult, Older Adult)
- Outcome measures - Clinical Deterioration|Change in Viral Load|Rate of Organ Failure|Progression to ICU Care or Ventilation|Number of Participants Who Had a Change in Clinical Status Measured by Decrease in COVID 7-point Ordinal Scale|Mortality|Rate of Severe Adverse Events|Number of Patients That Required Oxygen Supplementation|Number of Patients That Required Mechanical Ventilation|Number of Patients Who Required Vasopressors|Number of Patients Who Required ICU Services|Number of Patients That Required Hospitalization|Heart Function
|
|
NCT04385849
|
Study of the Safety of Therapeutic Treatment With an Immunomodulatory Agent (N-803) in Adults With COVID-19 |
Active, not recruiting |
Phase 1 |
Jul/22/2020 |
Jul/11/2022 |
- Alternative id - QUILT-COVID-19
- Interventions - Biological: N-803|Other: Saline
- Study type - Interventional
- Study results - No Results Available
- Locations - St. Francis, Lynwood, California, United States
- Study designs - Allocation: Randomized|Intervention Model: Parallel Assignment|Masking: Triple (Participant, Care Provider, Investigator)|Primary Purpose: Treatment
- Enrollment - 1
- Age - 18 Years and older (Adult, Older Adult)
- Outcome measures - Preliminary safety and efficacy evaluation of N-803 by adverse event (AE) incidence|Preliminary safety and efficacy evaluation of N-803 by subject clinical status using a the 7-point ordinal scale.|Preliminary safety and efficacy evaluation of N-803|Further evaluate efficacy of N-803 using changes to the National Early Warning Score (NEWS)|Further evaluate the safety of N-803 in hemoglobin|Further evaluate the safety of N-803 using platelets|Further evaluate the safety of N-803 using white blood cell count
|
|
NCT04255017
|
A Prospective/Retrospective,Randomized Controlled Clinical Study of Antiviral Therapy in the 2019-nCoV Pneumonia |
Unknown status |
Phase 4 |
Feb/01/2020 |
Jul/01/2020 |
- Alternative id - TJ20200128
- Interventions - Drug: Abidol hydrochloride|Drug: Oseltamivir|Drug: Lopinavir/ritonavir
- Study type - Interventional
- Study results - No Results Available
- Locations - Department and Institute of Infectious Disease, Wuhan, Hubei, China
- Study designs - Allocation: Randomized|Intervention Model: Parallel Assignment|Masking: Single (Participant)|Primary Purpose: Treatment
- Enrollment - 400
- Age - 18 Years and older (Adult, Older Adult)
- Outcome measures - Rate of disease remission|Time for lung recovery|Rate of no fever|Rate of respiratory symptom remission|Rate of lung imaging recovery|Rate of CRP,ES,Biochemical criterion(CK,ALT,Mb) recovery|Rate of undetectable viral RNA
|
|
NCT04582201
|
An Experiment to Evaluate the Safety of agenT-797 in COVID-19 Patients With Severe Difficulty Breathing. |
Recruiting |
Phase 1 |
Sep/21/2020 |
Jan/31/2023 |
- Alternative id - C-1300-01
- Interventions - Drug: agenT-797
- Study type - Interventional
- Study results - No Results Available
- Locations - Saint John's Cancer Institute, Santa Monica, California, United States|Norton Cancer Institute, St. Matthews Campus, Louisville, Kentucky, United States|Weill Cornell Medicine New York Presbyterian, New York, New York, United States
- Study designs - Allocation: N/A|Intervention Model: Single Group Assignment|Masking: None (Open Label)|Primary Purpose: Treatment
- Enrollment - 55
- Age - 18 Years and older (Adult, Older Adult)
- Outcome measures - Incidence of Treatment-Emergent Adverse Events|Number of Dose Limiting Toxicities|To assess time to improvement in pulmonary function.|Amount of virus detected in respiratory tract samples.|To assess longevity of agenT-797 infusion.
|
|
NCT04395859
|
Collateral Damage From the COVID-19 Pandemic Observed in Patients Treated With Intravitreal Injections (IVT) of Anti-angiogenic Agents |
Completed |
|
May/27/2020 |
Feb/15/2022 |
- Alternative id - MMT_2020_15
- Interventions - Procedure: Questionnaire|Other: Data collection up to 1 year
- Study type - Observational
- Study results - No Results Available
- Locations - Centre Pôle Vision du val d'Ouest, Ecully, France|Fondation Adolphe de Rothschild, Paris, France|Hôpital Lariboisière, Paris, France|Centre ophtalmologique Maison Rouge, Strasbourg, France
- Study designs - Observational Model: Cohort|Time Perspective: Prospective
- Enrollment - 233
- Age - 18 Years and older (Adult, Older Adult)
- Outcome measures - Change of visual acuity in patients treated with repeated IVT anti-angiogens during the COVID-19 epidemic
|
|
NCT04499677
|
FLARE: Favipiravir +/- Lopinavir: A RCT of Early Antivirals |
Completed |
Phase 2 |
Sep/24/2020 |
Dec/01/2021 |
- Alternative id - 132084
- Interventions - Drug: Favipiravir|Drug: Lopinavir/ Ritonavir|Other: Favipiravir Placebo|Other: Lopinavir/ Ritonavir Placebo
- Study type - Interventional
- Study results - No Results Available
- Locations - Royal Free Hospital, London, United Kingdom|University College London Hospital (UCLH), London, United Kingdom
- Study designs - Allocation: Randomized|Intervention Model: Factorial Assignment|Masking: Triple (Participant, Care Provider, Investigator)|Primary Purpose: Treatment
- Enrollment - 240
- Age - 18 Years to 70 Years (Adult, Older Adult)
- Outcome measures - Upper respiratory tract viral load at Day 5|Percentage of participants with undetectable upper respiratory tract viral load after 5 days of therapy|Proportion of participants with undetectable stool viral load after 7 days of therapy.|Rate of decrease in upper respiratory tract viral load during 7 days of therapy|Duration of fever following commencement of medication|Proportion of participants with hepatotoxicity after 7 days of therapy|Proportion of participants with other medication-related toxicity after 7 days of therapy and 14 days post-randomisation|Proportion of participants admitted to hospital with COVID-19 related illness|Proportion of participants admitted to ICU with COVID-19 related illness|Proportion of participants who have died with COVID-19 related illness|Pharmacokinetics of favipiravir as measured by Clearance (CL)|Pharmacokinetics of favipiravir as measured by Volume of distribution (V)|Pharmacokinetics of favipiravir as measured by Absorption rate constant (Ka)|Pharmacokinetics of favipiravir as measured by Maximum concentration (Cmax)|Pharmacokinetics of favipiravir as measured by Time to maximum concentration (Tmax)|Pharmacokinetics of favipiravir as measured by Elimination rate constant (Ke)|Pharmacokinetics of favipiravir as measured by Area Under the Curve extrapolated to infinity (AUC (0-inf)|Pharmacodynamics of favipiravir as measured by Rate of viral load decline (delta)|Pharmacodynamics of favipiravir as measured by Maximum increase in viral load under drug treatment (Emax)|Pharmacodynamics of favipiravir as measured by Concentration to achieve half the maximum possible effect (EC50)|Proportion of participants with deleterious or resistance-conferring mutations in SARS-CoV-2 by Day 7 of treatment
|
|
NCT04459702
|
A Study of Combination Therapies to Treat COVID-19 Infection |
Withdrawn |
Phase 2 |
Jul/01/2020 |
Dec/01/2021 |
- Alternative id - PRG-043
- Interventions - Drug: hydroxychloroquine|Drug: Azithromycin|Drug: Ritonavir|Drug: Lopinavir
- Study type - Interventional
- Study results - No Results Available
- Locations - ProgenaBiome, Ventura, California, United States
- Study designs - Allocation: Randomized|Intervention Model: Parallel Assignment|Masking: Double (Participant, Investigator)|Primary Purpose: Treatment
- Enrollment - 0
- Age - 18 Years to 65 Years (Adult, Older Adult)
- Outcome measures - Efficacy of Treatment by Reduced Symptoms NEWS (National Early Warning System) scores|Efficacy of Treatment by Time to Non-Infectivity|Safety of Dual Therapy as Measured by Symptoms rated on the NEWS (National Early Warning System) sores|Safety of Quadruple Therapy as Measured by Symptoms rated on the NEWS (National Early Warning System) scores.|Safety of Dual Therapy as Measured by Complete Blood Count|Safety of Quadruple Therapy as Measured by Complete Blood Count|Safety of Dual Therapy as Measured by Metabolic Panel -Albumin|Safety of Quadruple Therapy as Measured by Metabolic Panel - Albumin|Safety of Quadruple Therapy as Measured by Metabolic Panel - A/G Ratio|Safety of Dual Therapy as Measured by Metabolic Panel A/G Ratio|Safety of Quadruple Therapy as Measured by Metabolic Panel - Alkaline Phosphatase|Safety of Dual Therapy as Measured by Metabolic Panel Alkaline Phosphatase|Safety of Dual Therapy as Measured by Metabolic Panel - AST|Safety of Quadruple Therapy as Measured by Metabolic Panel - AST|Safety of Quadruple Therapy as Measured by Metabolic Panel - ALT|Safety of Dual Therapy as Measured by Metabolic Panel ALT|Safety of Dual Therapy as Measured by Metabolic Panel BUN/Creatinine Ratio|Safety of Quadruple Therapy as Measured by Metabolic Panel BUN/Creatinine Ratio|Safety of Quadruple Therapy as Measured by Metabolic Panel - BUN|Safety of Dual Therapy as Measured by Metabolic Panel - BUN|Safety of Dual Therapy as Measured by Metabolic Panel - Calcium|Safety of Quadruple Therapy as Measured by Metabolic Panel - Calcium|Safety of Quadruple Therapy as Measured by Metabolic Panel - Carbon Dioxide|Safety of Dual Therapy as Measured by Metabolic Panel - Carbon Dioxide|Safety of Dual Therapy as Measured by Metabolic Panel - Chloride|Safety of Quadruple Therapy as Measured by Metabolic Panel - Chloride|Safety of Quadruple Therapy as Measured by Metabolic Panel - Creatinine|Safety of Dual Therapy as Measured by Metabolic Panel - Creatinine|Safety of Dual Therapy as Measured by Metabolic Panel - Globulin|Safety of Quadruple Therapy as Measured by Metabolic Panel - Globulin|Safety of Quadruple Therapy as Measured by Metabolic Panel - Glucose|Safety of Dual Therapy as Measured by Metabolic Panel - Glucose|Safety of Dual Therapy as Measured by Metabolic Panel - Potassium|Safety of Quadruple Therapy as Measured by Metabolic Panel - Potassium|Safety of Quadruple Therapy as Measured by Metabolic Panel - Total Bilirubin|Safety of Dual Therapy as Measured by Metabolic Panel - Total Bilirubin|Safety of Dual Therapy as Measured by Metabolic Panel - Total Protein|Safety of Quadruple Therapy as Measured by Metabolic Panel - Total Protein|Safety of Dual Therapy as Measured by Treatment Related SAE|Safety of Quadruple Therapy as Measured by Treatment Related SAE
|
|
NCT04390152
|
Safety and Efficacy of Intravenous Wharton's Jelly Derived Mesenchymal Stem Cells in Acute Respiratory Distress Syndrome Due to COVID 19 |
Recruiting |
Phase 1|Phase 2 |
Jan/13/2020 |
Apr/01/2022 |
- Alternative id - BIOXSOMCOV001
- Interventions - Drug: Wharton's jelly derived Mesenchymal stem cells.|Drug: Hydroxychloroquine, lopinavir/ritonavir or azithromycin and placebo (standard therapy)
- Study type - Interventional
- Study results - No Results Available
- Locations - BioXcellerator, Medellin, Antioquia-CO, Colombia|Clinical Somer, Rionegro, Antioquia, Colombia
- Study designs - Allocation: Randomized|Intervention Model: Parallel Assignment|Masking: Quadruple (Participant, Care Provider, Investigator, Outcomes Assessor)|Primary Purpose: Treatment
- Enrollment - 40
- Age - 18 Years to 80 Years (Adult, Older Adult)
- Outcome measures - Intergroup mortality difference with treatment|Number of patients with treatment related adverse events|Difference in days of mechanical ventilation between groups|Median reduction of days of hospitalization|Median reduction of days of oxygen needs|Difference between "Sequential Organ Failure Assessment" score between groups|Difference between median Murray score between groups|Difference in APACHE II score between groups|Difference in lymphocyte count between groups|Changes in C reactive protein concentration between groups|Changes in D dimer concentration|Changes in ferritin concentration|Changes in lactate dehydrogenase concentration|Impact on interleukin 6 concentrations between groups.|Impact on interleukin 8 concentrations between groups.|Impact on interleukin 10 concentrations between groups.|Impact on tumor necrosis factor alpha concentrations between groups.
|
|
NCT04321174
|
COVID-19 Ring-based Prevention Trial With Lopinavir/Ritonavir |
Active, not recruiting |
Phase 3 |
Apr/17/2020 |
Mar/31/2022 |
- Alternative id - CORIPREV-1
- Interventions - Drug: Lopinavir/ritonavir
- Study type - Interventional
- Study results - No Results Available
- Locations - St. Paul's Hospital, Vancouver, British Columbia, Canada|Sunnybrook Hospital, Toronto, Ontario, Canada|St. Michael's Hospital, Toronto, Ontario, Canada|Toronto General Hospital, Toronto, Ontario, Canada
- Study designs - Allocation: Randomized|Intervention Model: Parallel Assignment|Masking: Single (Outcomes Assessor)|Primary Purpose: Prevention
- Enrollment - 123
- Age - 18 Months and older (Child, Adult, Older Adult)
- Outcome measures - Microbiologic evidence of infection|Adverse events|Symptomatic COVID-19 disease|Seropositivity|Days of hospitalization attributable to COVID-19 disease|Respiratory failure requiring ventilatory support attributable to COVID-19 disease|Mortality|Short-term psychological impact of exposure to COVID-19 disease|Long-term psychological impact of exposure to COVID-19 disease|Health-related quality of life
|
|
NCT05024006
|
Public Health Emergency: SOLIDARITY TRIAL Philippines |
Completed |
Not Applicable |
Apr/23/2020 |
Apr/17/2021 |
- Alternative id - SJREB-2020-20
- Interventions - Drug: Remdesivir|Drug: Hydroxychloroquine|Drug: Lopinavir / Ritonavir|Drug: Interferon beta-1a|Drug: Acalabrutinib
- Study type - Interventional
- Study results - No Results Available
- Locations - Baguio General Hospital, Baguio City, Benguet, Philippines|Cebu Doctor's University Hospital, Cebu City, Cebu, Philippines|Perpetual Succor Hospital Cebu, Cebu City, Cebu, Philippines|Vicente Sotto Memorial Medical Center, Cebu City, Cebu, Philippines|Southern Philippines Medical Center, Davao City, Davao, Philippines|West Visayas University Medical Center, Iloilo City, Iloilo, Philippines|Makati Medical Center, Makati City, Metro Manila, Philippines|Chinese General Hospital, Manila, Metro Manila, Philippines|Manila Doctors Hospital, Manila, Metro Manila, Philippines|ManilaMed - Medical Center Philippines, Manila, Metro Manila, Philippines|San Lazaro Hospital, Manila, Metro Manila, Philippines|UP - Philippine General Hospital, Manila, Metro Manila, Philippines|Asian Hospital and Medical Center, Muntinlupa, Metro Manila, Philippines|Research Institute for Tropical Medicine, Muntinlupa, Metro Manila, Philippines|San Juan de Dios Educational Foundation Inc - Hospital, Pasay, Metro Manila, Philippines|The Medical City, Pasig City, Metro Manila, Philippines|Diliman Doctors Hospital, Quezon City, Metro Manila, Philippines|Fe Del Mundo Medical Center, Quezon City, Metro Manila, Philippines|Lung Center of the Philippines, Quezon City, Metro Manila, Philippines|St Luke's Medical Center Quezon City, Quezon City, Metro Manila, Philippines|University of the East Ramon Magsaysay Memorial Medical Center, Quezon City, Metro Manila, Philippines|World Citi Medical Center, Quezon City, Metro Manila, Philippines|Cardinal Santos Medical Center, San Juan, Metro Manila, Philippines|St Luke's Medical Center Global, Taguig, Metro Manila, Philippines|Batangas Medical Center, Batangas, Philippines
- Study designs - Allocation: Randomized|Intervention Model: Parallel Assignment|Masking: None (Open Label)|Primary Purpose: Treatment
- Enrollment - 1314
- Age - 18 Years and older (Adult, Older Adult)
- Outcome measures - All-cause mortality|Duration of hospital stay|Time to first receiving ventilation
|
|
NCT04307693
|
Comparison of Lopinavir/Ritonavir or Hydroxychloroquine in Patients With Mild Coronavirus Disease (COVID-19) |
Terminated |
Phase 2 |
Mar/11/2020 |
Apr/30/2020 |
- Alternative id - S2020-0472-0001
- Interventions - Drug: Lopinavir/ritonavir|Drug: Hydroxychloroquine sulfate
- Study type - Interventional
- Study results - No Results Available
- Locations - Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea, Republic of
- Study designs - Allocation: Randomized|Intervention Model: Parallel Assignment|Masking: None (Open Label)|Primary Purpose: Treatment
- Enrollment - 65
- Age - 16 Years to 99 Years (Child, Adult, Older Adult)
- Outcome measures - Viral load|Viral load change|Time to clinical improvement (TTCI)|Percentage of progression to supplemental oxygen requirement by day 7|Time to NEWS2 (National Early Warning Score 2) of 3 or more maintained for 24 hours by day 7|Time to clinical failure, defined as the time to death, mechanical ventilation, or ICU admission|Rate of switch to Lopinavir/ritonavir or hydroxychloroquine by day 7|adverse effects|Concentration of Lopinavir/ritonavir and hydroxychloroquine
|
|
NCT04403100
|
Hydroxychloroquine and Lopinavir/ Ritonavir to Improve the Health of People With COVID-19: "The Hope Coalition - 1" |
Recruiting |
Phase 3 |
Jun/03/2020 |
Feb/01/2021 |
- Alternative id - COVID19_AMB_Brasil
- Interventions - Drug: Hydroxychloroquine Sulfate Tablets|Drug: Lopinavir/ Ritonavir Oral Tablet|Drug: Hydroxychloroquine Sulfate Tablets plus Lopinavir/ Ritonavir Oral Tablets|Drug: Placebo
- Study type - Interventional
- Study results - No Results Available
- Locations - CARDRESEARCH - Cardiologia Assistencial e de Pesquisa, Belo Horizonte, Minas Gerais, Brazil|Pontificia Universidade Catolica de Minas Gerais, Belo Horizonte, Minas Gerais, Brazil|Fundo Municipal de Saúde de Betim, Betim, Minas Gerais, Brazil|Universidade Federal de Ouro Preto, Ouro Preto, Minas Gerais, Brazil
- Study designs - Allocation: Randomized|Intervention Model: Factorial Assignment|Masking: Quadruple (Participant, Care Provider, Investigator, Outcomes Assessor)|Primary Purpose: Treatment
- Enrollment - 1968
- Age - 18 Years and older (Adult, Older Adult)
- Outcome measures - Proportion of participants who were hospitalized for progression of COVID-19 disease|Proportion of participants who died due to COVID-19 progression and/ or complications|Proportion of participants with viral load change on 03, 07, 10 and 14 after randomization|Time to clinical improvement|Time to clinical failure|Hospitalization for any cause|Proportion of participants who died due to pulmonary complications|Proportion of participants who died due to cardiovascular complications|Proportion of participants who presented with adverse events|Time to improvement on respiratory scale symptoms|proportion of non-adherent participants to any of study drugs
|
|
NCT04394182
|
Ultra Low Doses of Therapy With Radiation Applicated to COVID-19 |
Recruiting |
Not Applicable |
Apr/21/2020 |
Apr/21/2021 |
- Alternative id - 20.4.1597-GHM
- Interventions - Radiation: Ultra-Low-dose radiotherapy|Device: ventilatory support with oxygen therapy|Drug: Lopinavir/ritonavir|Drug: Hydroxychloroquine|Drug: Azithromycin|Drug: Piperacillin/tazobactam|Drug: Low molecular weight heparin|Drug: Corticosteroid injection|Drug: Tocilizumab
- Study type - Interventional
- Study results - No Results Available
- Locations - Hospital La Milagrosa, GenesisCare, Madrid, Spain|Hospital Vithas Valencia Consuelo, Valencia, Spain
- Study designs - Allocation: N/A|Intervention Model: Single Group Assignment|Masking: None (Open Label)|Primary Purpose: Supportive Care
- Enrollment - 15
- Age - 18 Years to 120 Years (Adult, Older Adult)
- Outcome measures - Oxygen Therapy Status at Day 2|Oxygen Saturation (Sat02; Pulse oximeter measurement) at Day 2|Blood Gas Analysis at Day 2|Blood Test at Day 2|Oxygen Therapy Status at Day 5|Oxygen Saturation (Sat02; Pulse oximeter measurement) at Day 5|Blood Test at Day 5|Oxygen Therapy Status at Day 7|Oxygen Saturation (Sat02; Pulse oximeter measurement) at Day 7|Blood Test at Day 7|Change from baseline Total Severity Score (TSS) analyzed in a thoracic CT scan at Day 7|Recovery time|COVID-19 status|Change from baseline Total Severity Score (TSS) analyzed in a thoracic CT scan al Month 1|Acute Toxicity
|