Almitrine
A respiratory stimulant.
General information
Almitrine is a respiratory stimulant that improves oxygenation via carotid body chemoreceptor activation (NCIt).
Almitrine on DrugBank
Almitrine on PubChem
Almitrine on Wikipedia
Marketed as
DUXIL; VECTARION
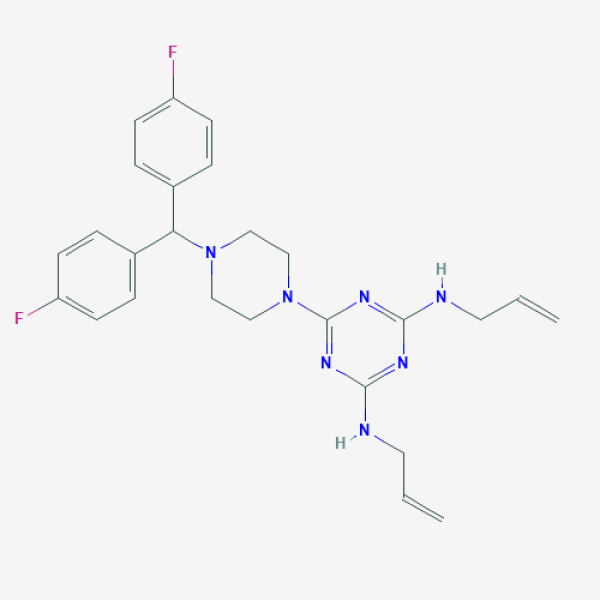
C=CCNC1=NC(=NC(=N1)N2CCN(CC2)C(C3=CC=C(C=C3)F)C4=CC=C(C=C4)F)NCC=C
Supporting references
| Link | Tested on | Impact factor | Notes | Publication date |
|---|---|---|---|---|
|
Almitrine as a non ventilatory strategy to improve intrapulmonary shunt in COVID-19 patients
|
Patients | 2.71 | almitrine infusion is associated with improved oxygenation |
Jun/04/2020 |
|
Identification of potential treatments for COVID-19 through artificial intelligence-enabled phenomic analysis of human cells infected with SARS-CoV-2
Preprint |
human renal cortical epithelial cells | strong effect |
Apr/23/2020 | |
|
"Almitrine Infusion in Severe Acute Respiratory Syndrome Coronavirus 2-Induced Acute Respiratory Distress Syndrome A Single-Center Observational Study"
ARDS Small molecule Cohort study |
Patients with ARDS | 7.41 | Improvement in oxygenation in some COVID-19 patients with ARDS. Sample size: 32. Dosage: 1.65 ± 0.4 μg/kg/min (mean) for 2 days (median). |
Oct/21/2020 |
AI-suggested references
Clinical trials
| ID | Title | Status | Phase | Start date | Completion date |
|---|---|---|---|---|---|
| NCT04380727 | Almitrine and COVID-19 Related Hypoxemia | Completed | Mar/20/2020 | Apr/25/2020 | |
|
|||||
| NCT04357457 | Efficacy of Intravenous Almitrine in Reducing the Need for Mechanical Ventilation in Patients With Hypoxemic Acute Respiratory Failure Due to Covid-19-related Pneumonia | Active, not recruiting | Phase 3 | Sep/03/2020 | Oct/01/2021 |
|
|||||
| NCT05216575 | Almitrine in COVID-19 Patients With ARDS Treated by HFNO | Recruiting | Jan/01/2021 | Mar/31/2022 | |
|
|||||
