(5-Chloropyridin-3-yl) 1H-indole-4-carboxylate
An anti-coronaviral protease inhibitor.
General information
(5-Chloropyridin-3-yl) 1H-indole-4-carboxylate is a chloropyridyl ester-derived SARS-CoV and SARS-CoV-2 3C-like protease covalent inhibitor with antiviral activities in micromolar concentrations in vitro (Ghosh et al, 2008; Hattori et al., 2021).
(5-Chloropyridin-3-yl) 1H-indole-4-carboxylate on PubChem
Synonyms
GRL-0496; GRL-1720
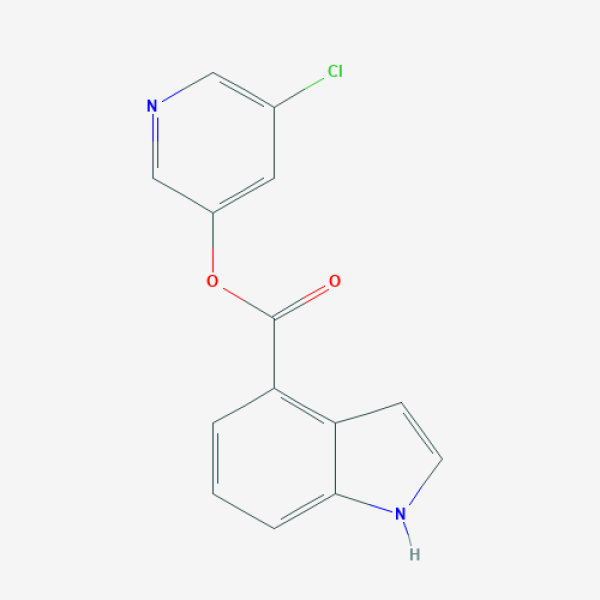
C1=CC(=C2C=CNC2=C1)C(=O)OC3=CC(=CN=C3)Cl
Supporting references
| Link | Tested on | Impact factor | Notes | Publication date |
|---|---|---|---|---|
|
Development of a Cell-Based Luciferase Complementation Assay for Identification of SARS-CoV-2 3CLpro Inhibitors
3CLpro Small molecule Enzyme assay In vitro |
in vitro enzyme assay | 3.82 | The compound inhibited the SARS-CoV-2 3C-like protease in vitro with maximum inhibition of 24% relative to the inhibition by 100 μM GC376 and it showed CC50 of 53.2 μM. |
Jan/24/2021 |
|
A small molecule compound with an indole moiety inhibits the main protease of SARS-CoV-2 and blocks virus replication
Crystallization Small molecule Enzyme assay In vitro In silico |
in silico; in vitro enzyme asay; in vitro biophysical assay; crystallization; Calu-3 cells, peripheral blood mononuclear cells, and human bronchial/tracheal epithelial cells (cytotoxicity assays); Vero E6 cells; SARS-CoV-2 strain JPN/TY/WK-521 | 12.12 | The compound potently inhibited the SARS-CoV-2 3C-like protease (3CLpro) in vitro (IC50 of ca. 0.32 μM) without detectable cytotoxicity at 200 μM. The compound’s EC50 value for Vero E6 cells was ca 15 μM. Using mass spectrometry and fluorimetry techniques, it was found to form a covalent bond with 3CLpro. |
Jan/28/2021 |
